What Is Brownian Motion? | Properties of Matter | Chemistry | FuseSchool
Summary
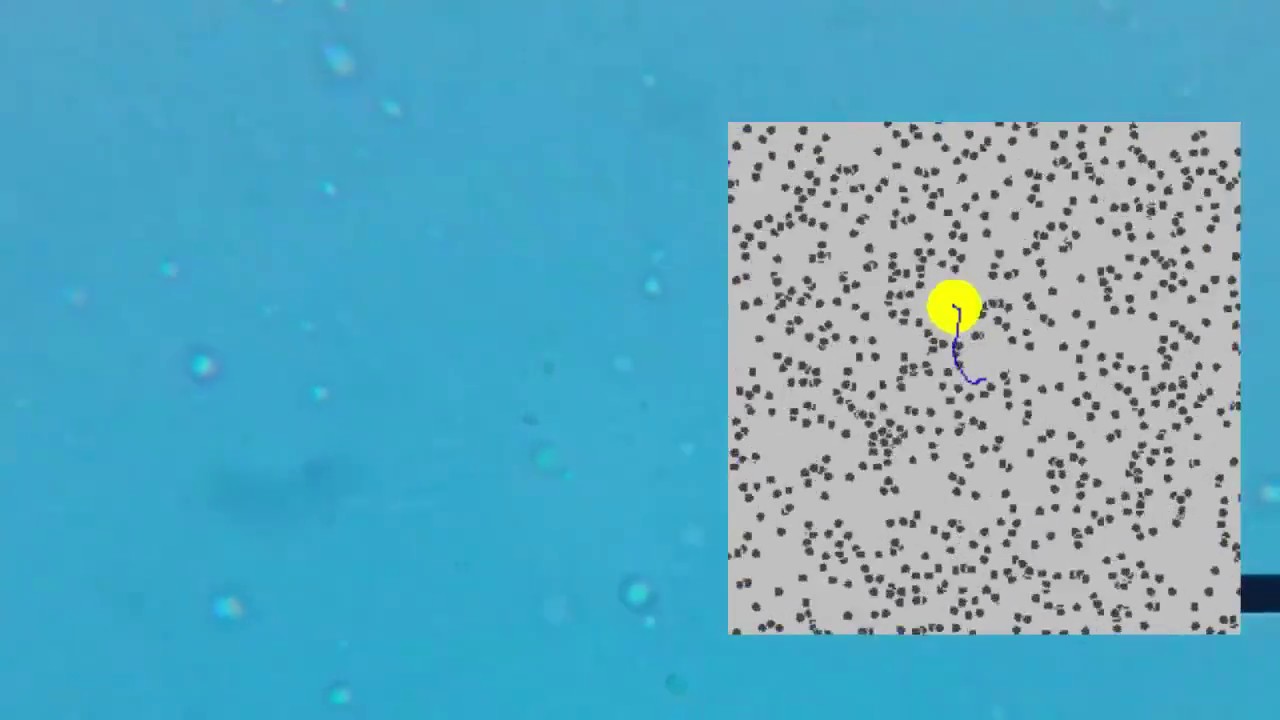
TLDRThis lesson explores Brownian motion, a phenomenon that indirectly confirmed the existence of atoms. It describes observing dust particles moving randomly in a beam of light due to collisions with unseen gas particles. The concept is further explained through the historical observation by Robert Brown of pollen grains 'jiggling' in water, which is now understood as the result of countless collisions with water molecules. Brownian motion illustrates the continuous and random movement of particles in fluids, whether gas or liquid.
Takeaways
- 🌪️ Brownian motion is the random movement of particles suspended in a fluid, like dust particles in air or pollen in water.
- 🕵️♂️ The observation of dust particles moving randomly in a beam of light is evidence of the existence of atoms, as the movement is caused by collisions with gas molecules.
- 🔍 Robert Brown first observed this phenomenon with pollen grains under a microscope, initially thinking the pollen was alive due to its movement.
- 🌬️ The fluid in which the particles are suspended can be a gas, like air, or a liquid, like water, and the particles' movement is due to collisions with the fluid's molecules.
- 🎲 The random direction changes of the dust particles are akin to a game of snooker, where many small particles collide with a larger, observable one.
- 🌐 The movement of dust particles is not always downwards but in all directions, indicating the chaotic nature of molecular collisions.
- 🤲 When we move our hand through the air, we feel the air particles' collisions against our hand, which is an indirect way of sensing the gas molecules.
- 🔬 Brownian motion indirectly confirms the existence of atoms, as the observed movement of larger particles is a result of countless unseen molecular collisions.
- 🧐 The challenge posed in the script asks us to consider why pollen grains appear to jiggle randomly, which is due to the same principle of molecular collisions as with dust particles.
- 💧 In the case of pollen grains in water, the liquid medium causes the observed Brownian motion, with water molecules too small to see but their effects visible on the pollen.
- 📚 Understanding Brownian motion helps in grasping the concept of atoms and molecules, and their behavior in different states of matter.
Q & A
What is Brownian motion?
-Brownian motion is the random movement of particles suspended in a fluid (liquid or gas) due to their collisions with the fluid's molecules.
Why do dust particles move randomly when light passes through them?
-Dust particles move randomly because they are constantly colliding with the air molecules, which are too small to see but cause the dust particles to change direction erratically.
What did Robert Brown observe when he was studying pollen grains under a microscope?
-Robert Brown observed that the pollen grains appeared to be jiggling or moving randomly, which was later understood to be due to the collisions with water molecules.
How does the movement of dust particles in a gas compare to the movement of pollen grains in water?
-Both dust particles in a gas and pollen grains in water exhibit random movement due to collisions with the molecules of the fluid they are suspended in, which is the essence of Brownian motion.
Why did Robert Brown initially think the pollen was alive?
-Robert Brown initially thought the pollen was alive because he saw the grains moving and jiggling, which he mistakenly attributed to life processes rather than the physical collisions with water molecules.
What can we infer about the size of gas particles from the script?
-We can infer that gas particles are too small to be seen directly because they are not visible to the naked eye, even though their effects on larger particles like dust can be observed.
How does the script describe the collisions between air particles and dust particles?
-The script describes the collisions as frequent and causing the dust particles to move off in new, random directions, similar to a game of snooker with many marbles colliding.
What is the significance of Brownian motion in confirming the existence of atoms?
-Brownian motion is significant because it indirectly confirms the existence of atoms by demonstrating the random, erratic movement of particles due to collisions with molecules, which are the building blocks of matter.
What happens when you move your hand through the air according to the script?
-When you move your hand through the air, you feel the air particles because they collide with your hand, demonstrating the presence of the gas.
Why is the movement of pollen grains in water considered Brownian motion?
-The movement of pollen grains in water is considered Brownian motion because the grains are suspended in the liquid and their random movement is caused by collisions with the water molecules.
How does the script illustrate the concept of many collisions between air particles and dust particles?
-The script uses the analogy of a game of snooker with millions of marbles to illustrate how frequent and random the collisions are, affecting the direction of the dust particles.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowBrowse More Related Video

Everyone should see this experiment for themselves
How do we know atoms are real? (Brownian Motion)

Brownian motion demonstration

The Nucleus: Crash Course Chemistry #1

TIDAK BISA DILIHAT BUKAN BERARTI TIDAK ADA!! FENOMENA INI MEMBUKTIKAN ATOM ITU NYATA!!

Brownian Motion - Defintion, Example, Experiment, Applications
5.0 / 5 (0 votes)