Brownian Motion - Defintion, Example, Experiment, Applications
Summary
TLDRThe video script explores Brownian motion, a phenomenon first observed by botanist Robert Brown, who noticed pollen grains moving erratically in a liquid. This random movement is due to the unequal bombardment of suspended particles by molecules in the surrounding medium. The script explains the difference in motion between large and small particles, with the latter experiencing a resultant force due to unequal impacts, leading to a zigzag path. An experiment involving a glass cube filled with particles under a microscope illustrates this molecular motion, providing visual evidence of Brownian motion.
Takeaways
- 🔬 Brownian motion is the random movement of tiny particles, such as pollen grains or colloidal particles, suspended in a liquid.
- 🌿 The phenomenon was first observed by English botanist Robert Brown, who noticed pollen grains moving erratically under a microscope.
- 🔍 This motion is caused by the unequal bombardment of suspended particles by the molecules of the surrounding liquid.
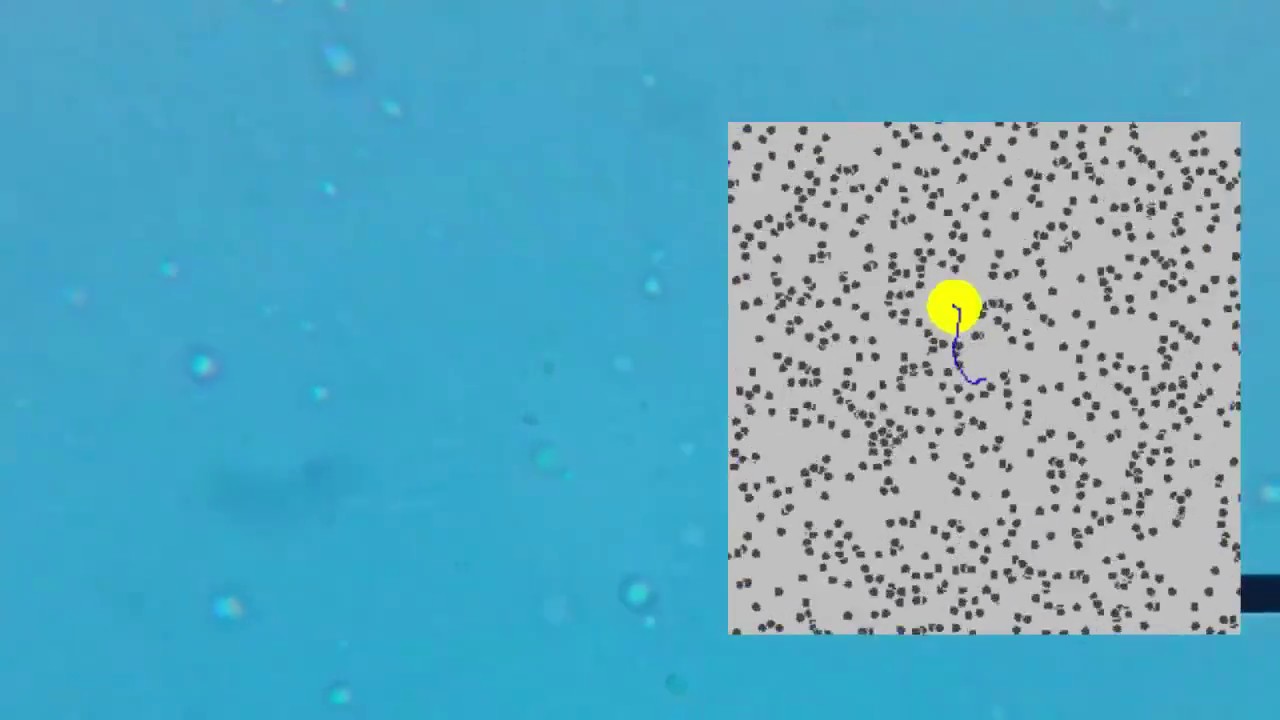
- 🧲 The resultant force from the impacts of these molecules on smaller particles, like particle B, causes changes in direction and a zigzag motion.
- 🔮 Large particles, like particle A, experience a more balanced impact from the surrounding molecules, resulting in no net force and less observable motion.
- 🧪 Brownian motion is a direct evidence of molecular motion, as the movement of particles is a response to the collisions with liquid molecules.
- 👨🔬 The script suggests an experiment involving a hollow glass cube filled with small particles, observed under a microscope to demonstrate Brownian motion.
- 💡 The experiment shows that particles appear as bright spots moving in various directions against a dark background, illustrating the continuous molecular activity.
- 🌟 The study of Brownian motion is significant as it provides insights into the kinetic theory of matter and the behavior of particles at the microscopic level.
- 📚 Understanding Brownian motion is fundamental to various fields of science, including physics, chemistry, and materials science.
Q & A
Who first provided evidence that matter consists of tiny particles in motion?
-The English botanist, Robert Brown, first provided evidence that matter consists of tiny particles in motion.
What did Robert Brown observe when studying pollen grains under a microscope?
-Robert Brown observed that pollen grains were dancing to and fro in a random manner, which is now known as Brownian motion.
What is the cause of the random motion observed in pollen grains and small colloidal particles?
-The random motion, or Brownian motion, is caused by the equal bombardment between the suspended particles and the molecules of the surrounding medium.
Why does an isolated large particle suspended in a solution not exhibit Brownian motion?
-An isolated large particle suspended in a solution does not exhibit Brownian motion because the impacts from the surrounding molecules mutually cancel out, resulting in a net force of zero.
How does the motion of a small particle like particle B differ from a large particle?
-A small particle like particle B is hit by fewer molecules and not equally from all sides, leading to a resultant force that changes its direction, causing a zigzag motion.
What happens to the direction of the resultant force on particle B as it moves?
-As particle B moves, the direction of the resultant force also changes, which results in the zigzag motion characteristic of Brownian motion.
What is the purpose of filling a hollow glass cube with small particles for the experiment?
-The purpose of filling a hollow glass cube with small particles is to observe Brownian motion under a microscope, where the particles appear as bright spots moving in all directions against a dark background.
How does the observation of smoke particles in the glass cube provide evidence of molecular motion?
-The observation of smoke particles moving in all possible directions within the glass cube under a microscope provides experimental evidence of molecular motion, as the particles are being jostled by the molecules in the air.
What is the significance of Brownian motion in understanding the nature of matter?
-Brownian motion is significant as it provides empirical evidence for the kinetic theory of matter, demonstrating that particles are in constant, random motion due to molecular collisions.
How can the experiment with the hollow glass cube be used to demonstrate Brownian motion to students?
-The experiment with the hollow glass cube can be used to demonstrate Brownian motion by showing students the movement of particles under a microscope, illustrating the concept of molecular motion.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowBrowse More Related Video
5.0 / 5 (0 votes)