Brownian motion demonstration
Summary
TLDRThis educational video demonstrates Brownian motion, illustrating its significance in confirming the existence of atoms and molecules. Using a simple suspension of polystyrene spheres in water, the presenter shows students the jiggling motion of these particles under a microscope. To further clarify the concept, a model involving a loudspeaker and table tennis balls simulates molecular collisions, allowing students to visualize the effects of invisible water molecules on a larger particle. This engaging demonstration emphasizes the random motion caused by countless smaller particles, enhancing understanding of this fundamental scientific phenomenon.
Takeaways
- 😀 Brownian motion provides indirect evidence for the existence of atoms and molecules.
- 😀 The phenomenon can be demonstrated in the lab using smoke cells or polystyrene spheres in water.
- 😀 A smoke cell is created by filling a capsule with smoke and observing it under a microscope.
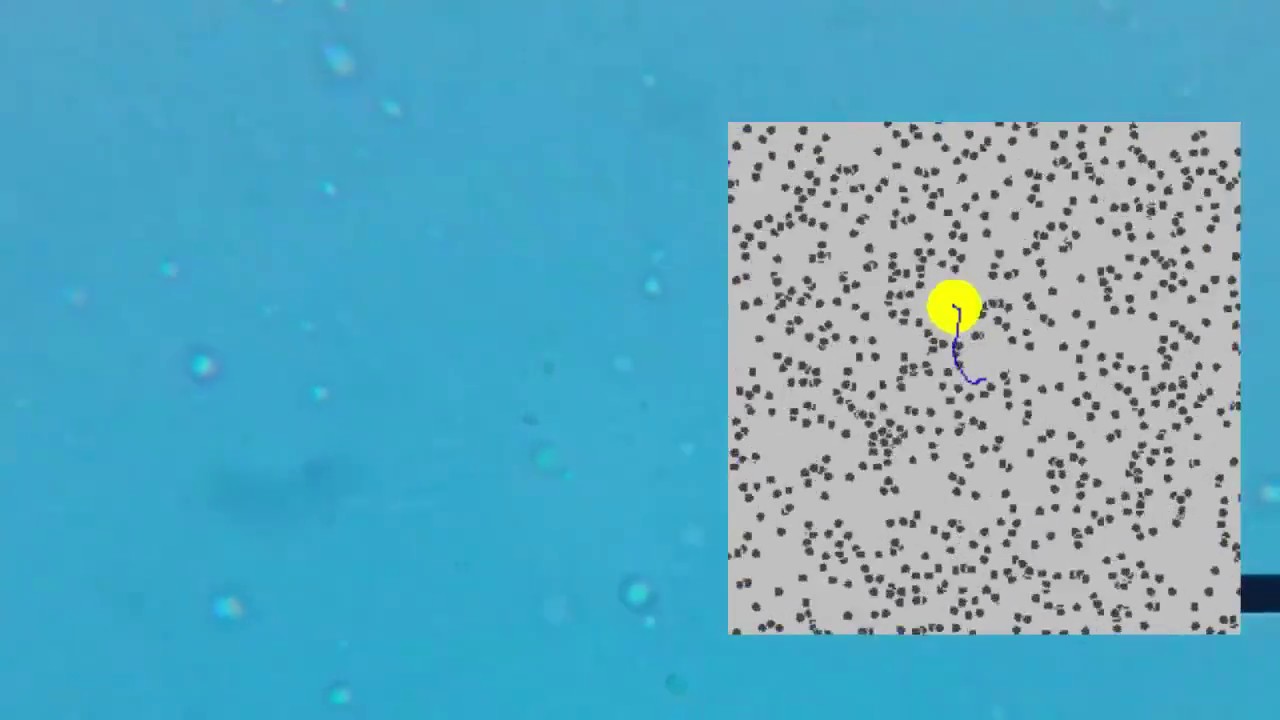
- 😀 Polystyrene spheres are used as a more convenient method to visualize Brownian motion.
- 😀 It's important for students to understand that they are observing polystyrene spheres, not atoms or molecules.
- 😀 The jiggling motion of the spheres is caused by collisions with water molecules, which are too small to see.
- 😀 A model using a loudspeaker and table tennis balls helps illustrate the concept of particle collisions.
- 😀 The demonstration shows how larger particles, like a balloon, move due to the random motion of smaller particles.
- 😀 Students should focus on the balloon's movement to grasp the analogy of Brownian motion.
- 😀 The classroom demonstration ensures all students observe the same experiment simultaneously.
Q & A
What is Brownian motion?
-Brownian motion refers to the random movement of particles suspended in a fluid, resulting from their collisions with fast-moving molecules in the fluid.
Why is Brownian motion significant in the study of physics?
-It provides indirect evidence for the existence of atoms and molecules, supporting foundational concepts in atomic theory.
Who were the key scientists involved in the analysis of Brownian motion?
-Albert Einstein and Marian Smoluchowski are two key figures who contributed to the understanding of Brownian motion.
What is a smoke cell, and how is it used in demonstrating Brownian motion?
-A smoke cell is a device filled with smoke from burning paper, viewed under a microscope to observe the random motion of smoke particles.
What alternative method is mentioned for demonstrating Brownian motion?
-The instructor uses a suspension of polystyrene spheres in deionized water as an alternative to the traditional smoke cell.
How does the instructor connect a microscope to enhance the demonstration?
-The microscope is connected to a video camera via an eyepiece adapter, allowing all students to observe the motion simultaneously.
What role do the table tennis balls play in the instructor's demonstration?
-The table tennis balls simulate water molecules, demonstrating how they collide with and cause movement in larger particles, represented by a balloon.
What analogy does the instructor use to help students understand Brownian motion?
-The instructor uses the analogy of a balloon being jostled by invisible table tennis balls to represent how larger particles move due to collisions with smaller ones.
What is the purpose of adjusting the voltage in the demonstration?
-Adjusting the voltage changes the amplitude of vibrations, which helps illustrate the random movement of particles in the fluid.
Why is it important for students to understand that they are not observing atoms or molecules directly?
-Students should recognize that they are seeing polystyrene spheres and that their motion is due to interactions with much smaller, invisible water molecules, which are responsible for Brownian motion.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowBrowse More Related Video

Everyone should see this experiment for themselves

The Nucleus: Crash Course Chemistry #1

What Is Brownian Motion? | Properties of Matter | Chemistry | FuseSchool
How do we know atoms are real? (Brownian Motion)

SISTEM KOLOID (Part 2), Sifat-sifat Sistem Koloid

TIDAK BISA DILIHAT BUKAN BERARTI TIDAK ADA!! FENOMENA INI MEMBUKTIKAN ATOM ITU NYATA!!
5.0 / 5 (0 votes)