Determination of Copper by Atomic Absorption Spectrophotometry
Summary
TLDRThis video demonstrates the process of copper determination in water using an Atomic Absorption Spectrophotometer (AAS). It starts with the preparation of a copper sulfate solution and follows through with the creation of standard solutions with varying concentrations of copper. The procedure includes precise measurements and dilution steps, culminating in the calibration and analysis of samples using the AAS. Detailed instructions on setting up the spectrophotometer and preparing the necessary reagents are provided, ensuring accuracy in the experiment for copper detection in water samples.
Takeaways
- 😀 The experiment determines copper concentration in water using Atomic Absorption Spectroscopy (AAS).
- 😀 Concentrated Nitric Acid (HNO₃) and Copper Sulfate (CuSO₄) are used to prepare stock solutions.
- 😀 A 100 ppm Cu stock solution is prepared by dissolving CuSO₄ in HNO₃ and deionized water, then diluted to 100 mL.
- 😀 A 10 ppm Cu standard solution is made by pipetting 10 mL of the 100 ppm stock solution into a 100 mL flask and diluting it with deionized water.
- 😀 A series of standard solutions with concentrations from 0 to 10 ppm Cu is prepared for calibration purposes.
- 😀 Sample preparation involves pipetting 2 mL of the water sample, diluting it to 100 mL with deionized water, and performing triplicate measurements for accuracy.
- 😀 The Atomic Absorption Spectrometer (AAS) is used to analyze the copper concentration by measuring absorption at a specific wavelength.
- 😀 The software Awin Lab is used to set up the AAS instrument, calibrate, and save the analysis method.
- 😀 The AAS spectrometer is calibrated using a linear regression method with known standard concentrations (0-10 ppm).
- 😀 The final concentration of copper in the water samples is determined by comparing sample absorption values with the calibration curve.
Q & A
What is the purpose of the experiment described in the script?
-The experiment aims to determine the copper (Cu) concentration in water using atomic absorption spectrophotometry (AAS).
What materials are used in the experiment?
-The materials used include concentrated HNO3 (nitric acid), CuSO4 (copper sulfate), distilled water (aquadest), and various laboratory equipment like spatulas, pipettes, and volumetric flasks.
How is the stock solution for copper sulfate prepared?
-To prepare the stock solution, 0.019 grams of CuSO4 is weighed and mixed with 0.6 mL of concentrated HNO3 and a small amount of distilled water. The mixture is transferred to a volumetric flask and diluted with distilled water to a final volume.
What is the purpose of creating the 100 PPM CuSO4 stock solution?
-The 100 PPM CuSO4 stock solution is used as a base to prepare standard solutions with varying copper concentrations for calibration in the spectrophotometer.
How are the standard solutions for calibration prepared?
-The standard solutions are prepared by pipetting 10 mL of the 100 PPM CuSO4 stock solution into a 100 mL volumetric flask and diluting it with distilled water to the mark. Different concentrations (0-10 PPM) are created by further dilution.
What steps are taken to prepare the sample for testing in the spectrophotometer?
-For testing, a 2 mL sample is pipetted into a volumetric flask and diluted to the mark with distilled water. The sample is then homogenized before analysis in the spectrophotometer.
What is the role of the atomic absorption spectrophotometer in the experiment?
-The atomic absorption spectrophotometer is used to measure the amount of absorbed light by the copper ions in the sample, which allows for the determination of copper concentration.
How is the spectrophotometer calibrated for the analysis?
-The spectrophotometer is calibrated by setting the parameters such as the lamp type and settings, as well as defining the method for analysis. The calibration is done using standard solutions with known copper concentrations (0-10 PPM).
What is the significance of using multiple sample repetitions in the experiment?
-Multiple repetitions of the sample (triplicates) are used to ensure accuracy and consistency in the results. This reduces the chance of error and provides a more reliable measurement of the copper concentration.
What should be done to avoid contamination during the sample preparation?
-To avoid contamination, it is important to handle the samples carefully, ensuring that no other materials come into contact with the solutions. Additionally, equipment should be cleaned properly, and the samples should be processed in a clean environment.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowBrowse More Related Video

Part 1 : Analisa Cu : Air Minum Dalam Kemasan (SNI 3553:2015) dengan Instrumen AAS Shimadzu AA7000

Metode pengujian logam Fe dengan AAS

Analisis Kadar Logam Besi (Fe) Pada Air Minum Dalam Kemasan Menggunakan Metode SSA

Quickly Understand Atomic Absorption Spectroscopy (AAS)
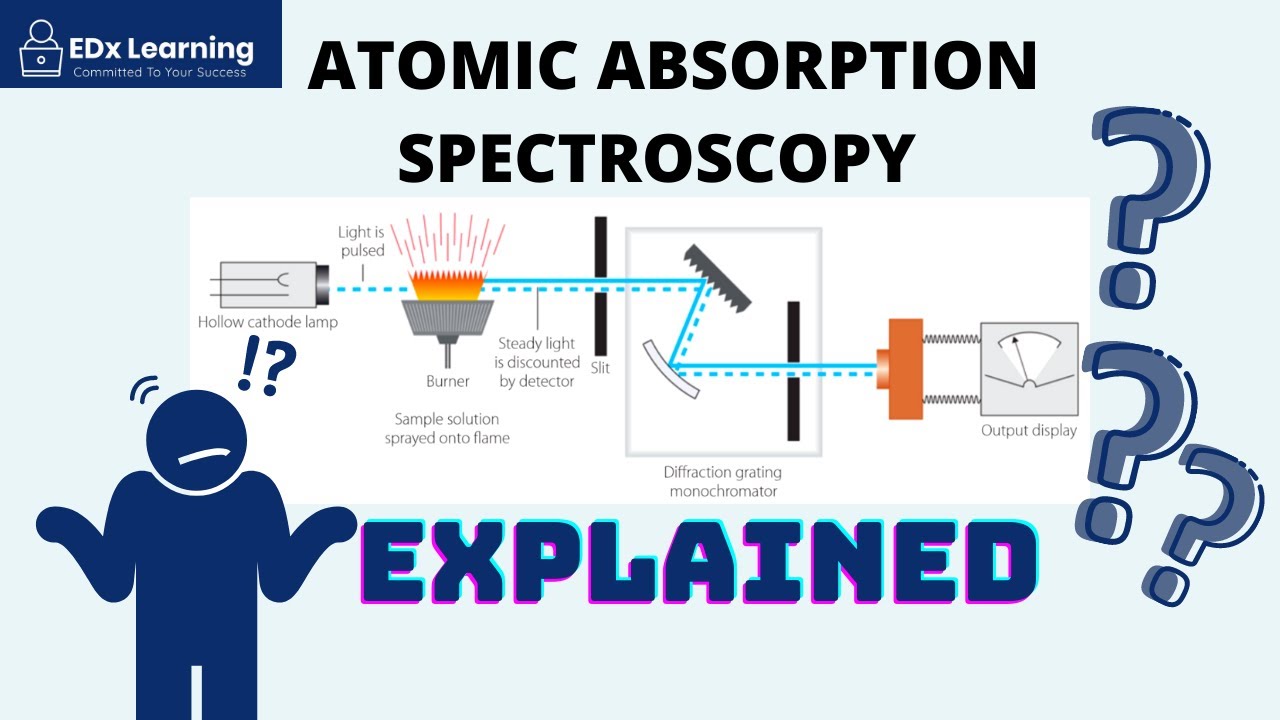
Atomic Absorption Spectroscopy (AAS) Explained - PART 1

Preparasi sampel untuk alat Atomic Absorption Spectrofotometry (AAS)
5.0 / 5 (0 votes)