How To Balance Chemical Equations
Summary
TLDRThis video teaches viewers how to balance chemical equations by adjusting coefficients. Using several examples, the video demonstrates how to balance atoms on both sides of the reaction, starting with elements that are less frequent. The process includes handling common issues such as unequal atom counts, fractions in coefficients, and the systematic approach for different types of reactions (e.g., combustion). Through clear, step-by-step guidance, the video helps learners grasp the core concepts of balancing equations in chemistry, providing practical solutions to common challenges in the process.
Takeaways
- 😀 Balancing chemical equations ensures that the number of atoms of each element is the same on both sides of the equation.
- 😀 Coefficients are added in front of reactants and products to balance the number of atoms.
- 😀 The least common multiple (LCM) method can be used when balancing atoms that do not match in number on both sides.
- 😀 For reactions involving sulfur, ensure that the number of sulfur atoms on both sides are the same by adjusting coefficients.
- 😀 When balancing chromium reactions, focus on balancing the chromium atoms after balancing other elements.
- 😀 Oxygen atoms in decomposition reactions should be balanced last, as they are usually easier to adjust after other elements.
- 😀 In combustion reactions, it’s best to start by balancing carbon atoms, followed by hydrogen, and finally oxygen atoms.
- 😀 Fractional coefficients may occur during balancing; multiplying the entire equation by the denominator of the fraction helps eliminate them.
- 😀 For reactions like the combustion of propane, adjusting oxygen atoms requires balancing carbon dioxide and water molecules first.
- 😀 In complex reactions, such as ammonia reacting with oxygen, carefully balance nitrogen and hydrogen atoms before finalizing oxygen atom balance.
Q & A
Why is it necessary to balance chemical equations?
-Balancing chemical equations ensures that the law of conservation of mass is upheld, meaning that the number of atoms of each element is the same on both sides of the equation.
What is the first step in balancing a chemical equation?
-The first step is to identify and balance the atoms of elements that appear in only one reactant and one product, starting with those that are least complex.
How do you balance sulfur atoms in the equation Cr + S → Cr₂S₃?
-To balance sulfur, first find the least common multiple of 8 (sulfur atoms on the left) and 3 (on the right), which is 24. Then, place a 3 in front of sulfur on the left and an 8 in front of Cr₂S₃ on the right.
What role do coefficients play when balancing chemical equations?
-Coefficients are numbers placed in front of chemical formulas to indicate how many molecules or atoms of a substance are involved, ensuring the number of atoms on both sides of the equation is equal.
Why is it important to balance the chromium atoms in the Cr + S → Cr₂S₃ reaction?
-It's important to balance chromium atoms to ensure that the number of chromium atoms is the same on both sides of the equation, maintaining the principle of conservation of mass.
What strategy can you use when balancing combustion reactions?
-For combustion reactions, balance carbon atoms first, followed by hydrogen atoms, and then balance oxygen atoms last. This method helps simplify the process and makes it easier to find the correct coefficients.
How do you balance oxygen atoms in the combustion of propane (C₃H₈)?
-To balance oxygen atoms in the combustion of propane, count the oxygen atoms on the right side (6 from CO₂ and 4 from H₂O), giving a total of 10. Then, place a 5 in front of O₂ on the left to balance the oxygen.
In the equation 2HgO → 2Hg + O₂, how is mercury balanced?
-Mercury is balanced by placing a 2 in front of both HgO on the left and Hg on the right, ensuring there are 2 mercury atoms on both sides of the equation.
Why is it necessary to multiply through by 2 when balancing the ammonia reaction 4NH₃ + 3O₂ → 2N₂ + 6H₂O?
-Multiplying through by 2 is necessary to eliminate the fraction (5/2) in the oxygen coefficient, ensuring all coefficients are whole numbers and the equation is properly balanced.
What happens if you encounter a fraction while balancing an equation?
-If you encounter a fraction, you can multiply all the coefficients by the denominator of the fraction to convert them into whole numbers, making the equation easier to balance.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowBrowse More Related Video

Penyetaraan Persamaan Reaksi Kimia dengan Cepat- Kimia Kelas 10
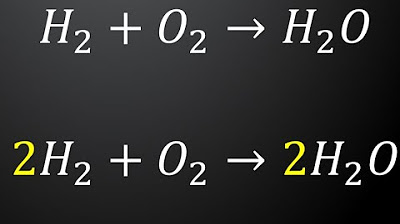
Chemistry: Balancing Chemical Equations (Tagalog Explained)

Cara mudah menyetarakan reaksi kimia - kimia SMA kelas 10 semester 2

BALANCEAMENTO QUÍMICO POR TENTATIVA

REACCIONES ORGANICAS DE COMBUSTIÓN | Ejercicios con Alcanos

Balancing Chemical Equations Step by Step Practice Problems | How to Pass Chemistry
5.0 / 5 (0 votes)