HUKUM DALTON | KIMIA "Tekanan Parsial"
Summary
TLDRThis video explains Dalton's Law of Partial Pressures through a hands-on demonstration using simple materials. It describes how the total pressure of a gas mixture equals the sum of the partial pressures of the individual gases. The experiment involves two bottles filled with colored water, connected by tubing. When the water mixes, it shows how the pressures in both bottles are balanced, proving Dalton's Law. The video is a practical demonstration that illustrates the concept of partial pressures in a visually engaging and easy-to-understand way.
Takeaways
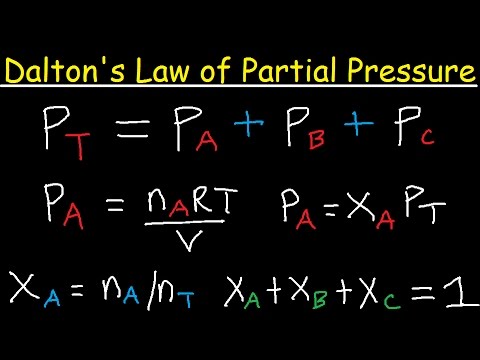
- 😀 Dalton's Law of Partial Pressures states that the total pressure of a gas mixture is equal to the sum of the partial pressures of the individual gases.
- 😀 The required materials for demonstrating Dalton's Law are simple and easy to gather, including a used plastic bottle, food coloring, a small tube, and some water.
- 😀 The plastic bottle needs to be cleaned before use and prepared with small holes in the cap, which are made by heating a nail.
- 😀 Two small tubes (about 1 meter long) are used to connect the bottles and allow air or liquid to flow between them.
- 😀 The bottles should be filled with water, which is separated into two portions of 600 mL and 300 mL.
- 😀 Different food colors (green and yellow) are added to the water in the bottles to easily distinguish between them during the experiment.
- 😀 The bottles are connected with tubes, and the water is filled and mixed to demonstrate how pressure can be measured in both bottles.
- 😀 The experimental setup works by showing that when two gases (represented by colored water) are at the same temperature and volume, their partial pressures are equal.
- 😀 A visual observation of the water flowing from one bottle to the other shows that the pressures in both bottles balance out, demonstrating Dalton’s Law.
- 😀 At equilibrium, no more water flows, proving that the total pressure is the sum of the individual partial pressures in each bottle, as per Dalton's Law.
Q & A
What is Dalton's Law of Partial Pressures?
-Dalton's Law of Partial Pressures states that the total pressure of a gas mixture is equal to the sum of the partial pressures of the individual gases in the mixture.
What materials are needed to create the demonstration tool for Dalton's Law?
-The materials required are an empty plastic bottle (such as a used mineral water bottle), food coloring (green and yellow), plastic, a small tube (approximately one meter long), a heated nail for making holes in the bottle cap, and approximately 900 mL of water, divided into two portions of 600 mL and 300 mL.
How are the holes in the bottle cap created for the demonstration?
-The holes in the bottle cap are created by heating a nail with a candle and then using it to make two holes in the cap, one for each tube.
What is the role of the plasticine in this demonstration?
-The plasticine is used to seal the area around the tube inside the bottle to prevent water from leaking out.
How is the water prepared for the experiment?
-The water is divided into two portions: 600 mL and 300 mL. The 600 mL is mixed with green food coloring, while the 300 mL is mixed with yellow food coloring. Both portions are stirred until the colors are fully mixed.
What does the setup represent in Dalton's Law demonstration?
-In the demonstration, the two bottles (one containing green water and the other yellow water) represent two separate gases. The water colors signify different gases in the mixture, and their interaction shows how partial pressures work together to form the total pressure.
What happens when the two bottles are tilted during the experiment?
-When the bottles are tilted, the colored water flows from one bottle to the other, demonstrating that the pressures in both bottles balance out. This action shows how the pressures from both gases contribute to the total pressure.
What is the significance of the water height in each bottle during the experiment?
-Before mixing, the water levels in the bottles are different, indicating varying partial pressures. After mixing, the water levels in both bottles equalize, demonstrating that the pressures from both gases (represented by the different colored waters) combine to form a total pressure.
How does the experiment demonstrate Dalton's Law mathematically?
-Dalton's Law is represented by the formula: P_total = P_A + P_B. In the experiment, the heights of the water columns in both bottles, when combined, represent the total pressure, showing that the total pressure is the sum of the partial pressures from each gas.
What conclusion can be drawn from the balanced water levels after the experiment?
-The balanced water levels in both bottles indicate that the partial pressures from the two gases (represented by the colored water) are equal at equilibrium. This supports Dalton's Law, where the total pressure is the sum of the individual partial pressures of the gases.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowBrowse More Related Video
Dalton's Law of Partial Pressure Problems & Examples - Chemistry

Dalton's Law

Graham's Law of Diffusion, Dalton's Law of Partial Pressure

Gas Law Formulas and Equations - College Chemistry Study Guide

Vapor Pressure | Raoult's Law | Solution Class 12

HUKUM PERBANDINGAN BERGANDA ( HUKUM DALTON ) : HUKUM DASAR KIMIA KELAS 10
5.0 / 5 (0 votes)