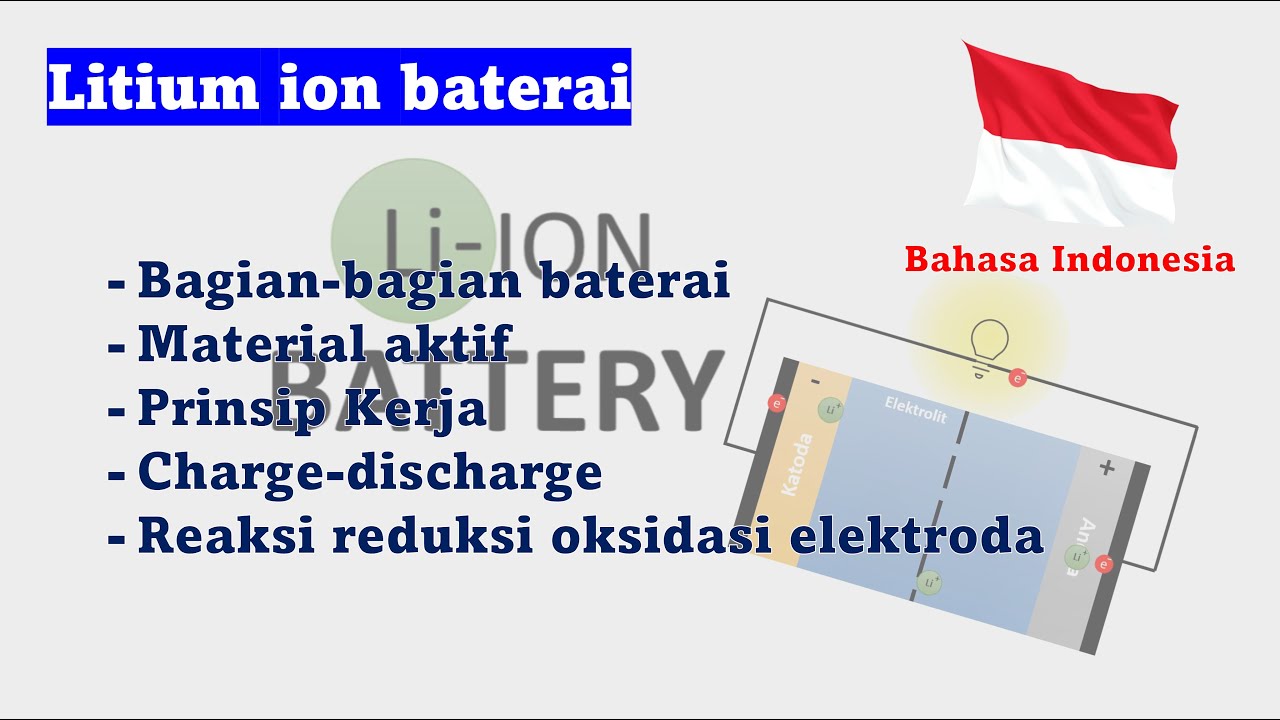
Working of Li Ion Battery | Function of Li-ion Battery
Summary
TLDRThis video explains the working of lithium-ion batteries and their applications, emphasizing the importance of electrochemical reactions in energy conversion. The speaker discusses the role of electrolytes, voltage, and chemical processes like oxidation and reduction in battery charging and discharging cycles. Various types of rechargeable batteries are mentioned, with a focus on their energy density and efficiency. Additionally, the video highlights the practical uses of these batteries in power systems and other technologies, encouraging viewers to subscribe for more informative content on the topic.
Takeaways
- 😀 Lithium-ion batteries are essential for modern electronics due to their high energy density and rechargeable nature.
- 😀 The battery's electrochemical properties convert chemical energy into electrical energy, powering devices.
- 😀 The charging process involves lithium ions moving from the cathode to the anode, storing energy.
- 😀 During discharging, lithium ions return from the anode to the cathode, releasing energy as electrical power.
- 😀 Oxidation and reduction reactions play a vital role in the battery's charging and discharging processes.
- 😀 Electrolytes, often in liquid form, facilitate the movement of ions between the battery’s electrodes.
- 😀 The energy density of lithium-ion batteries directly impacts the performance and longevity of devices.
- 😀 Common applications of lithium-ion batteries include smartphones, laptops, electric vehicles, and other portable electronics.
- 😀 Higher energy density in lithium-ion batteries means longer-lasting power for gadgets and improved efficiency.
- 😀 Recharging a lithium-ion battery involves restoring energy by reversing the ion flow that occurs during discharge.
Q & A
What is the primary focus of the video?
-The video primarily focuses on explaining the working principles of lithium-ion batteries, including their electrochemical properties, charging and discharging cycles, and applications in various power systems.
What are the main electrochemical processes mentioned in the script?
-The main electrochemical processes discussed include charging, discharging, reduction, and oxidation reactions, which occur within the battery during energy conversion and storage.
What role do electrolytes play in lithium-ion batteries?
-Electrolytes in lithium-ion batteries serve as a medium to facilitate the movement of ions between the positive and negative electrodes, enabling the chemical reactions necessary for charging and discharging.
What is the significance of 'energy density' in rechargeable batteries?
-Energy density refers to the amount of energy a battery can store relative to its size or weight. A higher energy density allows a battery to store more power, which is critical for applications requiring long-lasting and high-performance batteries.
Why is charging and discharging crucial for battery performance?
-Charging and discharging cycles are vital for maintaining battery performance. During charging, the battery stores energy, while during discharging, it releases the stored energy to power devices. The efficiency of these processes affects the battery's lifespan and energy output.
What does 'reduction' and 'oxidation' refer to in the context of battery operation?
-'Reduction' refers to the gain of electrons by a substance during the electrochemical reaction, while 'oxidation' refers to the loss of electrons. These reactions occur at the positive and negative electrodes of a battery during charging and discharging.
How do acids affect the performance of lithium-ion batteries?
-The script mentions that acids, such as those used in electrolytes, can enhance the performance of lithium-ion batteries by improving their energy density. The acidic environment helps optimize the electrochemical reactions that store and release energy.
What are some common applications of lithium-ion batteries mentioned?
-Lithium-ion batteries are commonly used in power systems, portable electronic devices, and electric vehicles due to their high energy density and efficiency in energy storage and conversion.
How do battery modes like 'battery saver' and 'charging mode' affect battery use?
-Battery saver mode reduces the energy consumption of a device to extend battery life, while charging mode restores energy to the battery. Switching between these modes helps manage power usage based on the device’s needs.
What does the phrase 'subscribe' refer to in the context of the video script?
-The repeated mention of 'subscribe' in the script seems to be a promotional message encouraging viewers to subscribe to the channel for more content related to the discussed topics, such as battery technology and electrochemical processes.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowBrowse More Related Video
5.0 / 5 (0 votes)