Logarithms and the pH scale
Summary
TLDRThis presentation explains the concept of logarithms, focusing on base 10 logarithms and their real-world applications, particularly in the pH scale. It covers how logarithms represent exponents, demonstrates the relationship between logarithmic increases and 10-fold changes in numbers, and introduces negative logarithms. The presentation also explains how the pH scale uses negative logarithms to measure hydrogen ion concentration, illustrating the inverse relationship between pH and acidity. By the end, viewers will understand how logarithms are key to understanding both mathematical principles and the behavior of acids and bases in chemistry.
Takeaways
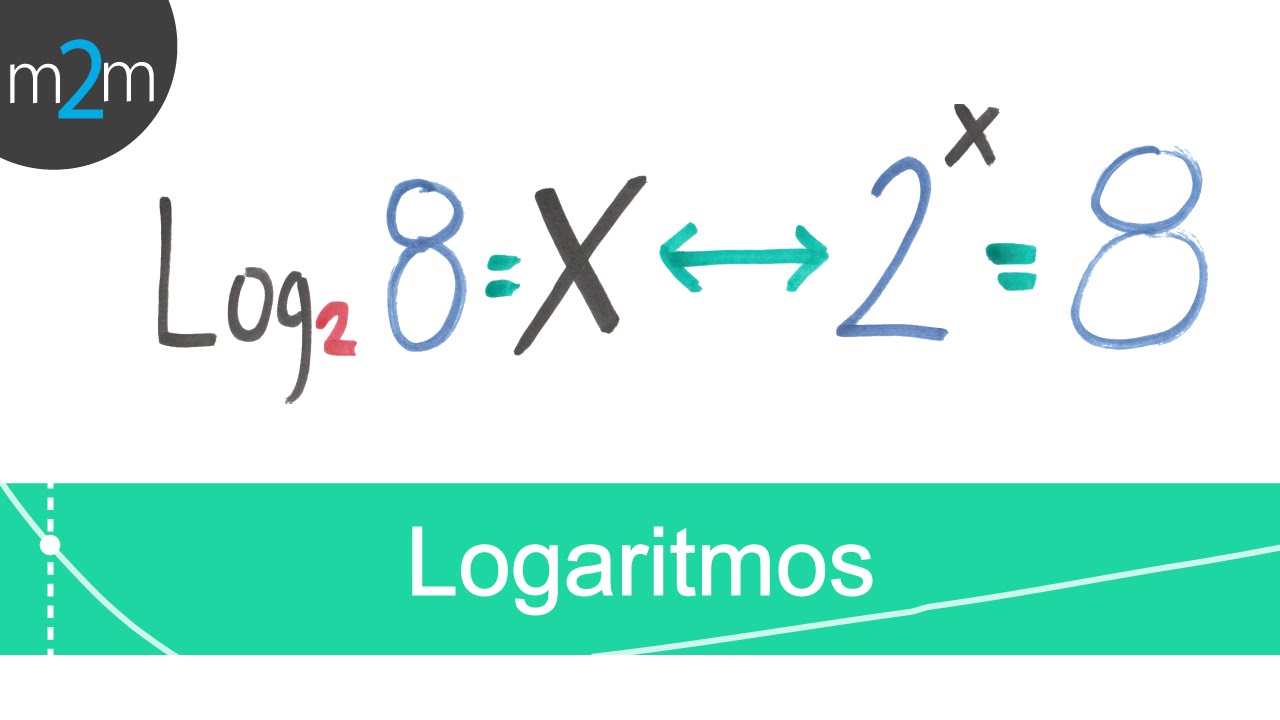
- 😀 Logarithms are the inverse of exponentiation, showing how many times a number must be multiplied to reach another number.
- 😀 A logarithm can be written as an exponent, such as log₁₀(1000) = 3, meaning 10 must be multiplied three times to get 1000.
- 😀 Base 10 logarithms (common logarithms) are most commonly used, but logarithms can also be written for other bases, such as base 2.
- 😀 In base 2, log₂(16) = 4, since 2 × 2 × 2 × 2 = 16.
- 😀 A 1-unit increase in log₁₀ corresponds to a 10-fold increase in the number. For example, log₁₀(100) = 2 and log₁₀(1000) = 3.
- 😀 Negative logarithms show how many times you must divide a number by 10 to get another value. For example, log₁₀(0.001) = -3.
- 😀 Logarithms have practical implications, such as how large numbers (like 10⁶ or 10¹²) are represented more easily with logarithms.
- 😀 The pH scale, used to measure the acidity or alkalinity of a solution, is based on negative base-10 logarithms of hydrogen ion concentration.
- 😀 When the pH decreases by 1 unit, the hydrogen ion concentration increases 10-fold, showing the logarithmic nature of the scale.
- 😀 The relationship between the pH value and the concentration of hydrogen ions explains why a lower pH means more acidity (higher H⁺ concentration).
Please replace the link and try again.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowBrowse More Related Video
5.0 / 5 (0 votes)