Lab Experiment #13: The Equilibrium Constant.
Summary
TLDRThis video tutorial demonstrates how to experimentally determine the equilibrium constant for the iron(III) and thiocyanate reaction to form iron(III) thiocyanate using Beer-Lambert Law and a spectrophotometer. The presenter explains the significance of using colored solutions for absorbance measurements, guiding viewers through sample preparation, including dilutions and mixing. By measuring the absorbance at 446 nm, participants can calculate the concentration of the complex ion and subsequently derive the equilibrium constant. The experiment emphasizes the importance of maintaining consistent conditions to ensure accurate results.
Takeaways
- 😀 The experiment aims to determine the equilibrium constant using the Beer-Lambert law and a spectrophotometer.
- 😀 The reaction involves iron (III) and thiocyanate to form the colored complex iron thiocyanate.
- 😀 The only significant colored species in the solution is the iron thiocyanate complex, which absorbs light maximally at 446 nm.
- 😀 The molar absorptivity for the complex ion is 4860 L/(mol·cm).
- 😀 The equilibrium constant expression is based on the concentrations of reactants and products at equilibrium.
- 😀 Initial concentrations of reactants must be calculated after preparing dilutions, not just taken from the bottles.
- 😀 Increasing thiocyanate concentration shifts the equilibrium to produce more iron thiocyanate complex, darkening the solution.
- 😀 Absorbance can be measured to find the concentration of the complex ion using the Beer-Lambert law.
- 😀 The equilibrium constant should remain constant across different samples if the temperature is unchanged.
- 😀 The experiment requires careful preparation of samples, including mixing and resting to ensure equilibrium is reached.
Q & A
What is the main objective of the experiment discussed in the transcript?
-The main objective is to experimentally determine the equilibrium constant for the reaction between iron(III) ions and thiocyanate ions using the Beer-Lambert Law and a spectrophotometer.
Why were iron(III) ions and thiocyanate ions chosen for this experiment?
-They were chosen because both reactants are colorless or very pale in color, making the resulting iron thiocyanate complex the only colored species in the solution, which is essential for measuring absorbance.
What is the significance of the absorbance maximum at 446 nanometers?
-The absorbance maximum at 446 nanometers indicates the wavelength at which the iron thiocyanate complex absorbs light most effectively, allowing for accurate measurements using the spectrophotometer.
How is the equilibrium constant (K) expressed mathematically?
-The equilibrium constant K is expressed as K = [Fe(SCN)²⁺] / ([Fe³⁺][SCN⁻]), where the concentrations are taken at equilibrium.
What role does the Beer-Lambert Law play in this experiment?
-The Beer-Lambert Law relates the absorbance of the solution to the concentration of the absorbing species, allowing for the calculation of the concentration of the iron thiocyanate complex.
How is the concentration X of the iron thiocyanate complex determined?
-X is calculated using the formula X = A / (ε × L), where A is the absorbance measured, ε is the molar absorptivity, and L is the path length of the cuvette.
What is the procedure for preparing the samples in the experiment?
-Each sample involves adding a fixed amount of iron(III) solution (5 mL), varying amounts of water and thiocyanate solution, then mixing and allowing the solution to reach equilibrium.
Why is it important to perform dilutions when preparing the solutions?
-Dilutions are important to achieve the desired concentrations of the reactants, as the initial concentrations listed on the bottles may not reflect what is actually used in the experiment.
What should remain constant when calculating the equilibrium constant for all samples?
-The temperature should remain constant, as it is the only factor that affects the equilibrium constant.
What happens to the color of the solution as more thiocyanate is added?
-As more thiocyanate is added, the solution darkens in color, indicating the formation of more iron thiocyanate complex.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowBrowse More Related Video

Lab - The Equilibrium Constant
Experiment 41.1 Effect of changing concentration on equilibrium position

Kimia Dasar 2 : Kesetimbangan (Spektrofotometer UV-VIS)

Hukum Dasar Kimia ( Latihan Soal Hukum Perbandingan Tetap/Hukum Proust) - Kimia
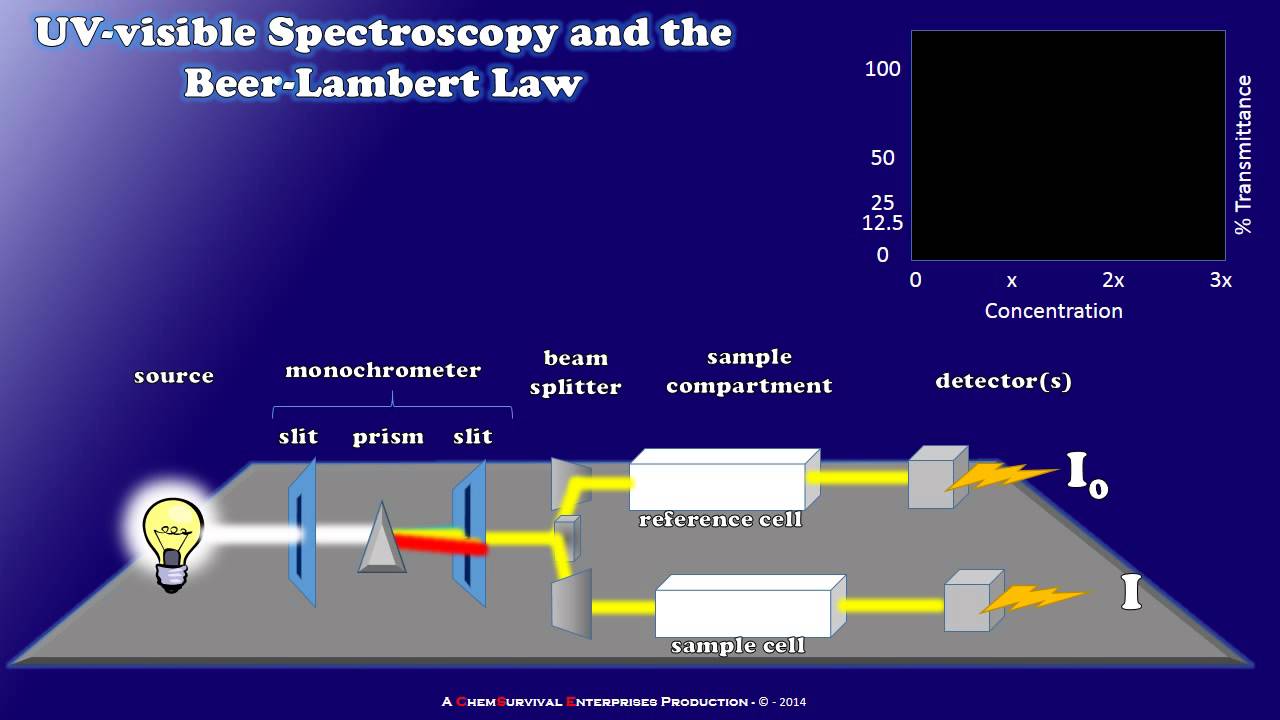
How a Simple UV-visible Spectrophotometer Works

Precipitation Titration: Mohr's & Volhard's Method // HSC Chemistry
5.0 / 5 (0 votes)