Lab - The Equilibrium Constant
Summary
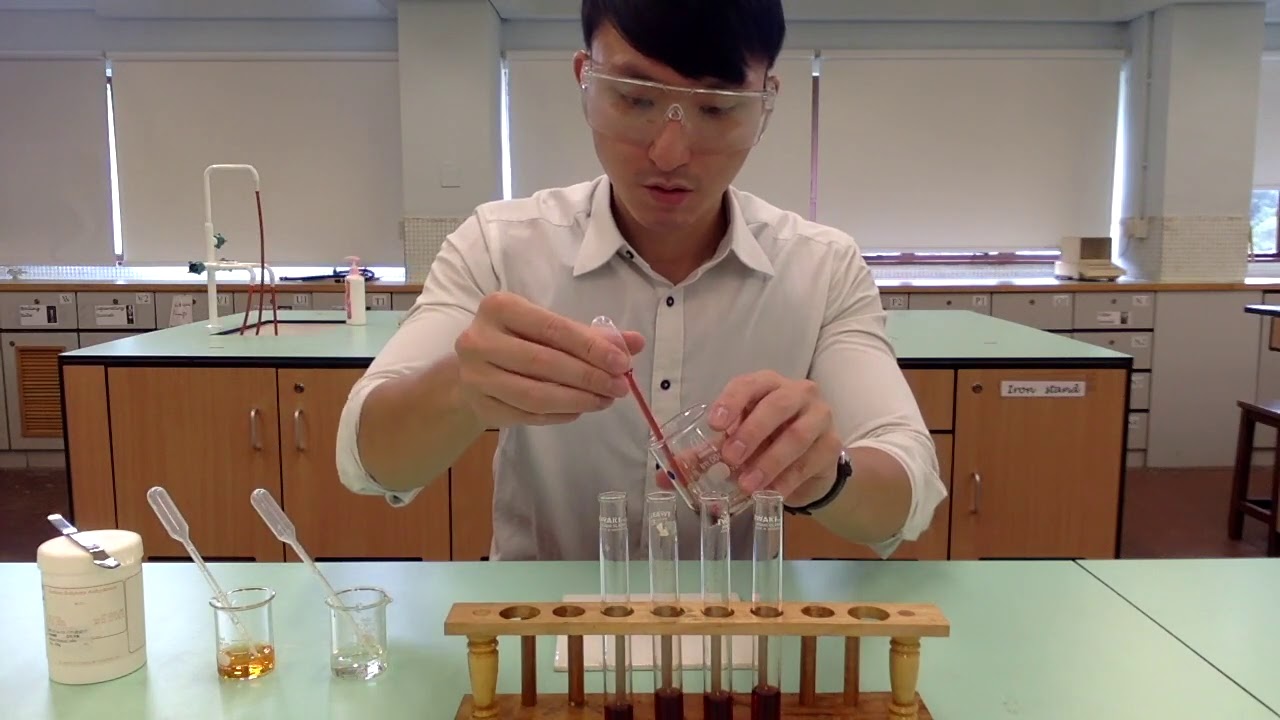
TLDRIn this experiment, we determine the equilibrium constant for the reaction between iron 3 ions and thiocyanate ions to form a red-colored FeSCN2+ complex. By measuring the absorption of the red complex using a spectrophotometer, we create reference solutions to establish a correlation between concentration and absorbance. We then use this reference data to measure unknown solutions at equilibrium, calculate the concentrations, and determine the equilibrium constant. The experiment involves five trials with different concentrations of iron and thiocyanate ions to ensure accuracy and consistency of results.
Takeaways
- 😀 The equilibrium constant (K_eq) describes the position of equilibrium for reversible reactions.
- 😀 The reaction in the experiment involves iron(III) ions and thiocyanate ions forming a red-colored FeSCN2+ complex.
- 😀 The concentration of FeSCN2+ is proportional to the intensity of the red color, which can be measured using a spectrophotometer.
- 😀 The equilibrium constant expression involves the concentration of FeSCN2+ as the numerator, and the product of the concentrations of Fe3+ and SCN- as the denominator.
- 😀 In Part A of the experiment, reference solutions are created to generate known concentrations of FeSCN2+ for calibration.
- 😀 Le Chatelier's principle is applied in Part A, where excess Fe3+ ensures the complete conversion of thiocyanate ions to FeSCN2+.
- 😀 Spectrophotometric measurements are taken at 417 nm, a wavelength in the blue region, as red liquids absorb blue light.
- 😀 A standard curve is developed by measuring absorbance at various known concentrations of FeSCN2+ to establish a correlation.
- 😀 Part B of the experiment involves mixing equal quantities of Fe3+ and SCN- ions to determine the equilibrium constant (K_eq) at equilibrium.
- 😀 Multiple test trials are conducted, with slightly varying concentrations, to ensure accurate and consistent results for calculating K_eq.
Q & A
What is the main objective of this experiment?
-The main objective is to determine the equilibrium constant for the reaction between iron 3 ions (Fe3+) and thiocyanate ions (SCN-) that forms a red FeSCN2+ complex ion.
Why is the FeSCN2+ complex ion important in this experiment?
-The FeSCN2+ complex ion is important because it is red in color, and the intensity of the red color is proportional to the concentration of the complex ion, allowing us to measure its concentration using a spectrophotometer.
How does the equilibrium constant expression for the reaction look?
-The equilibrium constant expression is given by the concentration of FeSCN2+ in the numerator, and the product of the concentrations of Fe3+ and SCN- in the denominator.
How is the equilibrium constant determined in this experiment?
-The equilibrium constant is determined by mixing known concentrations of Fe3+ and SCN-, measuring the resulting FeSCN2+ concentration at equilibrium using a spectrophotometer, and then using these values in the equilibrium constant expression.
What role does Le Châtelier's principle play in Part A of the experiment?
-Le Châtelier's principle helps in ensuring that the excess Fe3+ ions convert all of the thiocyanate ions into FeSCN2+, allowing for accurate measurement of the FeSCN2+ concentration.
Why do we need to create reference solutions in Part A?
-Reference solutions are created to establish a correlation between the concentration of FeSCN2+ and its absorbance, which will be used to determine the concentration of FeSCN2+ in unknown solutions.
What is the significance of the spectrophotometer in this experiment?
-The spectrophotometer is used to measure the absorbance of light at 417 nanometers, which correlates to the concentration of the red FeSCN2+ complex in the solution.
Why is distilled water used in the spectrophotometer as a blank?
-Distilled water is used as a blank to calibrate the spectrophotometer, ensuring that any absorbance measurements taken are only due to the sample solutions and not the cuvette or other factors.
What do we expect to observe during Part A of the experiment when measuring the absorbance of the reference solutions?
-In Part A, as we measure the absorbance of the reference solutions, we expect to observe a direct correlation between the concentration of FeSCN2+ and the absorbance, with darker red solutions showing higher absorbance values.
How does the experiment ensure accuracy when calculating the equilibrium constant?
-Accuracy is ensured by performing five separate test trials with slightly different concentrations of Fe3+ and SCN-, which should lead to nearly identical values for the equilibrium constant (Keq) across all trials.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowBrowse More Related Video

Lab Experiment #13: The Equilibrium Constant.
Experiment 41.1 Effect of changing concentration on equilibrium position

Kimia - ANIMASI PERCOBAAN PENGARUH KONSENTRASI DAN VOLUME PADA PERGESERAN KESETIMBANGAN

Precipitation Titration: Mohr's & Volhard's Method // HSC Chemistry

Calcium and Magnesium ion concentration determination with EDTA titration

Magnesium Oxide and water| Acids & Bases | Chemistry
5.0 / 5 (0 votes)