The law of conservation of mass - Todd Ramsey
Summary
TLDRThis script explores the origin of atoms that constitute all matter, from rocks and cows to our hearts, through the lens of the law of conservation of mass. It illustrates how atoms rearrange in chemical reactions without mass loss, and delves into the Big Bang's role in creating hydrogen. It further explains how stars fuse lighter elements into heavier ones, releasing energy as per Einstein's equation, and how supernovas disperse these elements across the universe, eventually forming Earth and all living beings, affirming we are indeed made of 'star stuff'.
Takeaways
- 🌌 The law of conservation of mass states that matter and energy cannot be created or destroyed in an isolated system.
- 🧪 Atoms bond to form molecules, and through energy, they can recombine, but the total amount of mass remains constant.
- 💧 Water and carbon dioxide are examples of molecules formed by the rearrangement of atoms.
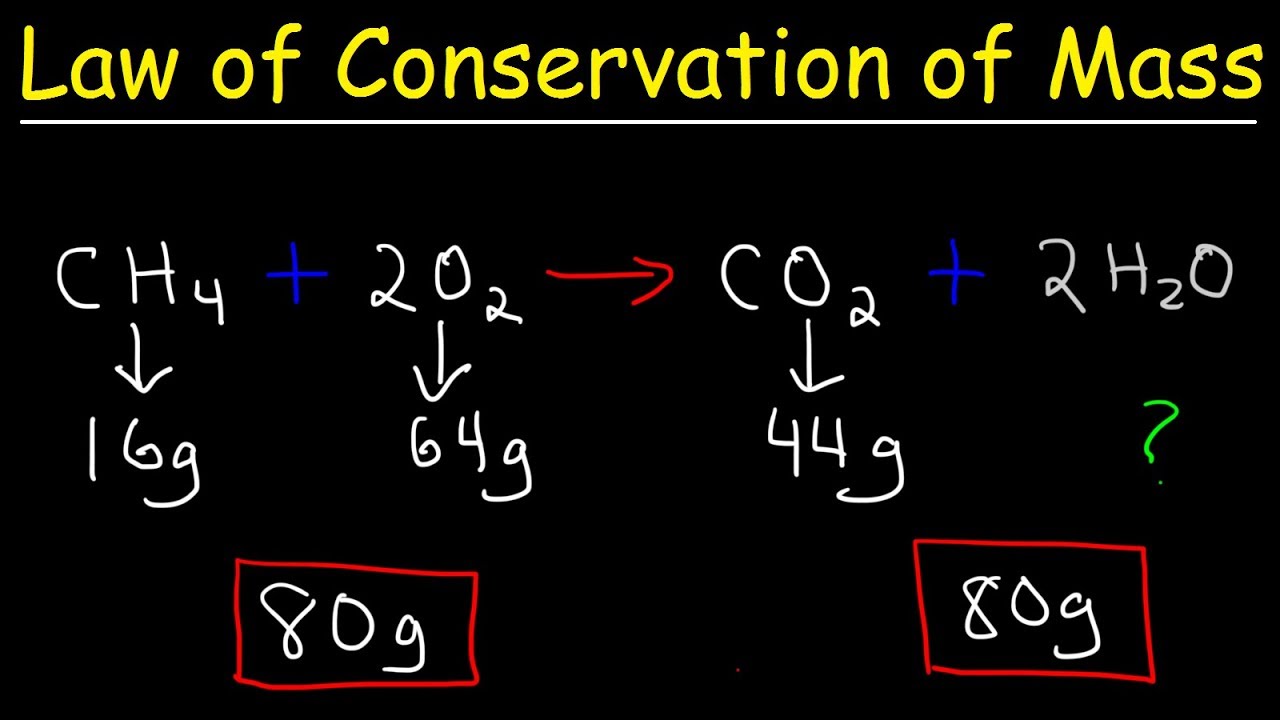
- 🔥 Combustion reactions, like burning methane or propane, still conserve the original atoms, but release energy in the process.
- 🔬 Chemical reactions follow the law of conservation of mass, ensuring that the matter and energy involved are always accounted for.
- 🕰 The atoms that make up everything today originated from the Big Bang and subsequent nuclear reactions in stars.
- 🌟 Stars fuse lighter elements like hydrogen and helium into heavier elements, such as carbon and oxygen, during their lifecycle.
- ⚛️ The mass lost during nuclear fusion in stars is converted into energy, as described by Einstein's equation (E=mc²).
- 💥 Supernovas scattered elements across the universe, and these elements eventually formed Earth and living organisms.
- 🌠 Carl Sagan famously said, 'We are all made of star stuff,' emphasizing that the atoms in our bodies were forged in stars.
Q & A
What is the law of conservation of mass?
-The law of conservation of mass states that in an isolated system, matter and energy can neither be created nor destroyed. This means that the total amount of mass and energy in the system remains constant, regardless of the processes that occur within it.
How does the law of conservation of mass apply to chemical reactions?
-In chemical reactions, the number and type of atoms remain the same before and after the reaction. The atoms may rearrange to form different molecules, but the total mass is conserved, meaning all atoms are accounted for at the end of the reaction.
What happens when atoms combine to form molecules?
-When atoms combine, they form chemical bonds, creating new molecules. For example, two hydrogen atoms and one oxygen atom form water, while a combination of carbon and oxygen atoms forms carbon dioxide.
What role does energy play in chemical reactions?
-Energy is required to break bonds in the reactants and form new bonds in the products. This energy is stored in the chemical bonds between atoms, and can be released or absorbed depending on the reaction.
What happens during the combustion of methane?
-During the combustion of methane, it reacts with oxygen in the presence of energy (e.g., from a lit match), forming carbon dioxide and water. This reaction releases a significant amount of energy in the form of heat and light.
How do nuclear reactions inside stars produce heavier elements?
-Inside stars, nuclear fusion reactions combine lighter elements, like hydrogen and helium, to form heavier elements such as carbon and oxygen. These reactions release a tremendous amount of energy and are the primary source of all elements in the universe beyond hydrogen and helium.
How does Einstein’s equation (E=mc²) relate to mass and energy in nuclear reactions?
-Einstein's equation shows that mass and energy are interchangeable. In nuclear reactions, some mass is converted into energy. The slight loss of mass during these reactions corresponds exactly to the amount of energy released, as described by E=mc².
What are supernovas and why are they important for element formation?
-Supernovas are massive stellar explosions that occur when a star exhausts its nuclear fuel and collapses. They scatter elements formed in the star’s core across space, enriching the surrounding interstellar medium and seeding new stars, planets, and even life.
How does the formation of Earth relate to the life cycle of stars?
-The Earth was formed from elements produced by previous generations of stars. These elements, scattered by supernovas, came together 4.6 billion years ago to form the Earth and other planets in the solar system.
What did Carl Sagan mean by 'we are all made of star stuff'?
-Carl Sagan's phrase means that the elements that make up our bodies, such as carbon, oxygen, and nitrogen, were formed in the interiors of stars. When these stars exploded, they spread these elements, which later formed new stars, planets, and ultimately life.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowBrowse More Related Video

What is the universe made of? - Dennis Wildfogel

Materi, Atom dan Sejarah Perkembangan Atom

S1.1.2 Physical and chemical changes
Law of Conservation of Mass - Fundamental Chemical Laws, Chemistry

Law of definite proportions | Atoms and Molecules | Chemistry | Khan Academy

Dalton's Atomic Theory || 3D Animated explanation || Complete Basics || Chemistry || Class 9th &11th
5.0 / 5 (0 votes)