States Of Matter - Solids, Liquids & Gases | Properties of Matter | Chemistry | FuseSchool
Summary
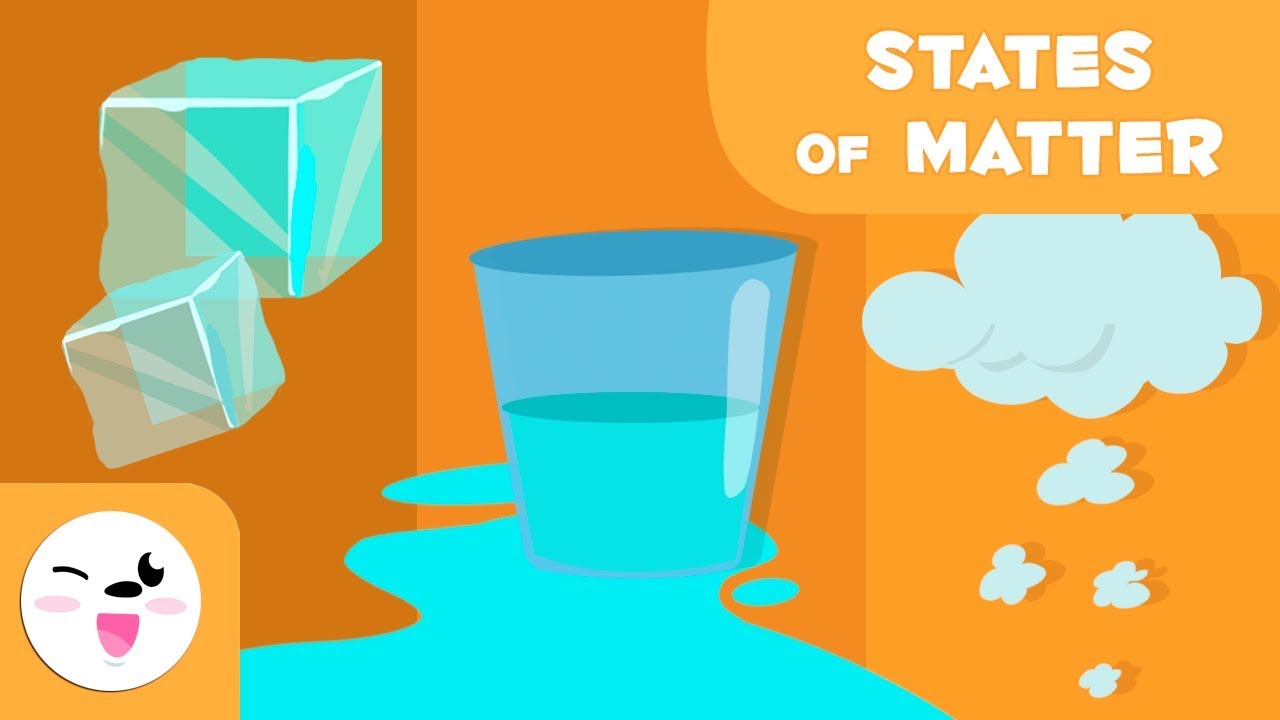
TLDRThis educational video explains the three states of matter: solid, liquid, and gas. Solids, like a computer, have closely packed, immobile particles, making them rigid with a fixed shape and volume. Liquids, such as water, have closely packed particles that can move, allowing flow but retaining volume, despite the lack of a fixed shape. Gases, exemplified by helium, have widely spaced particles, making them compressible and shapeless, filling any container they occupy.
Takeaways
- 🧊 The three states of matter are solid, liquid, and gas.
- 💻 Solids have particles closely packed in fixed positions, making them rigid with a fixed shape and volume.
- 💧 Liquids have particles closely packed but can move around each other, allowing flow but maintaining a fixed volume.
- 💡 A common misconception is that liquids do not have a fixed volume, but they do.
- 🍺 An example to illustrate liquid volume is ordering a pint of beer, which cannot be turned into two pints by changing containers.
- 🌬️ Gases have particles far apart, making them compressible and fill the entire space available without a fixed shape or volume.
- 🎈 Helium is an example of a gas used in party balloons due to its low density and unreactive nature.
- 🔍 The properties of each state of matter are dependent on how the particles are packed.
- 📏 Solids cannot be compressed or squashed due to their tightly packed particles.
- 🌊 Liquids do not have a fixed shape but can take the shape of their container while maintaining volume.
- 🌀 Gases are highly compressible and can be squashed or compressed easily.
Q & A
What are the three states of matter?
-The three states of matter are solid, liquid, and gas.
What is an example of a solid?
-An example of a solid is a computer.
How are the particles in solids arranged?
-The particles in solids are closely packed in fixed positions and cannot move around.
What are the characteristic properties of solids?
-Solids are rigid, cannot be squashed or compressed, have a fixed shape, and a fixed volume.
What is an example of a liquid?
-An example of a liquid is water.
How are the particles in liquids arranged compared to solids?
-The particles in liquids are closely packed but not as closely as in solids, allowing them to move around each other.
What are the characteristic properties of liquids?
-Liquids are not rigid, cannot be squashed or compressed, do not have a fixed shape, but have a fixed volume.
Why do liquids not have a fixed shape but do have a fixed volume?
-Liquids do not have a fixed shape because their particles can move over one another, but they have a fixed volume because the number of particles is constant and they occupy space.
What is an example of a gas?
-An example of a gas is helium, which is used in party balloons.
How are the particles in gases arranged compared to solids and liquids?
-The particles in gases are far apart and not closely packed, allowing gases to be compressible and to expand to fill any space.
What are the characteristic properties of gases?
-Gases are not rigid, can be squashed or compressed, do not have a fixed shape, and do not have a fixed volume; they fill the entire space they are in.
Why is it incorrect to say that liquids do not have a fixed volume?
-It is incorrect because, despite not having a fixed shape, liquids maintain a fixed volume as the total space occupied by the particles does not change.
How do the properties of matter relate to the arrangement of its particles?
-The properties of matter are directly related to how closely packed the particles are. In solids, they are tightly packed, in liquids they are less so but still closely, and in gases, they are far apart.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowBrowse More Related Video

CHANGES IN STATES OF MATTER || FREEZING, MELTING, CONDENSATION, EVAPORATION, SUBLIMATION, DEPOSITION

3 States of Matter - Solid, Liquid, Gas
States of matter for kids - What are the states of matter? Solid, liquid and gas

Matéria e energia [Módulo 01_Aula 01]

Science 5 Quarter 1 Week 5 Revised K-12 - Temperature And Its Effect On The State Of Matter

Zat dan Perubahannya | IPAS

States of Matter : Solid Liquid Gas
5.0 / 5 (0 votes)