🧪 Spectroscopie d'absorption UV-visible (avec @myMaxicours)
Summary
TLDRThis educational video script introduces young chemistry students to the use of a UV-visible spectrophotometer for absorption spectroscopy. It covers the basics of spectrophotometry, explaining the physical principles behind light absorption by chemical species and the importance of the Beer-Lambert law. The script guides viewers through practical demonstrations, including setting up the spectrophotometer, preparing samples, and interpreting absorption spectra. It also touches on the preparation of a copper sulfate solution and discusses the relationship between concentration and absorbance. The video is designed to be informative and engaging, with a focus on practical skills and theoretical understanding.
Takeaways
- 🔬 The video is a tutorial for young chemistry students on using a UV-Vis spectrophotometer to study chemical species.
- 🌟 The spectrophotometry technique is non-destructive, allowing for the analysis of a solution without altering it.
- 📊 It's used for various objectives such as identifying chemical characteristics, determining concentrations, and monitoring reactions.
- 🧪 The tutorial is in partnership with Maxicours, an educational platform, highlighting collaborative efforts in educational content creation.
- 📈 The principle of spectrophotometry is based on the interaction of matter with radiation, specifically how a chemical species absorbs light at certain wavelengths.
- 🌈 The video explains the concept of absorption, where a colored solution absorbs certain wavelengths of light, which is why objects appear colored.
- 🔍 The spectrophotometer works by shining white light through a sample, which is then split into monochromatic light, passed through the sample, and measured for absorbance.
- 💧 The tutorial emphasizes the importance of using clean cuvettes without air bubbles and the correct type of cuvette material for different wavelength ranges.
- 📉 The process of 'zeroing' or 'blanking' the spectrophotometer is crucial for accurate measurements, where the instrument is calibrated with a blank sample before the actual sample is measured.
- 📚 The video provides a practical demonstration of measuring absorbance at a fixed wavelength and tracing a full absorption spectrum for a copper sulfate solution.
- 📖 The tutorial concludes with an application of the Beer-Lambert law, which models the relationship between absorbance, concentration, and path length, and discusses its limitations.
Q & A
What is the purpose of the video?
-The purpose of the video is to teach young chemistry students how to use a UV-visible spectrophotometer, understand its functioning, and learn how to perform absorption measurements in a practical lab setting.
What is spectrophotometry and why is it used?
-Spectrophotometry is a non-destructive physical method that relies on the interaction between matter and radiation. It's used to analyze a sample by sending light through it and measuring the absorbed light, which provides information about the sample without altering it.
What is the significance of the absorption phenomenon in spectrophotometry?
-The absorption phenomenon is crucial in spectrophotometry because it allows for the measurement of how much light is absorbed by a chemical species at different wavelengths. This absorption is indicative of the chemical properties and concentration of the species in solution.
What is meant by the term 'absorbance' in the context of the video?
-Absorbance, denoted as 'A', is a physical quantity that measures the amount of light absorbed by a solution in relation to the light that passes through it. It is dimensionless and depends on the wavelength of light, the concentration of the absorbing species, and the optical path length.
How does a spectrophotometer work?
-A spectrophotometer works by using a light source that emits white light, which is then filtered through a monochromator to select a specific wavelength. The light passes through a cuvette containing the sample, and the transmitted light is detected and converted into an electrical signal, which is then recorded.
Why is it important to perform a 'blank' measurement in spectrophotometry?
-Performing a 'blank' measurement is essential to account for any absorption due to the solvent or cuvette itself. This ensures that the measured absorbance is solely due to the absorbing species in the sample, providing accurate and reliable results.
What is transmittance and how is it related to absorbance?
-Transmittance, denoted as 'T', is the fraction of light intensity that is transmitted through a sample. It is related to absorbance through the equation A = -log(T), where A is absorbance and T is transmittance. A higher transmittance means less absorption, and vice versa.
What is the significance of the maximum absorption wavelength in a spectrum?
-The maximum absorption wavelength in a spectrum indicates the wavelength at which the chemical species absorbs light most strongly. This information is crucial for identifying the species and for conducting precise measurements, as the spectrophotometer's sensitivity and accuracy are highest at this wavelength.
How does the concentration of a solution affect its absorbance?
-According to the Beer-Lambert Law, the absorbance of a solution is directly proportional to the concentration of the absorbing species and the path length of light through the solution. This means that a higher concentration will result in greater absorbance, assuming the solution is sufficiently dilute and the law is applicable.
What are the practical considerations when using cuvettes in spectrophotometry?
-When using cuvettes, it's important to ensure they are free of air bubbles, not overfilled, and contain a clear solution without suspended particles. The cuvette material should be appropriate for the wavelength range of interest, and the optical faces should be clean and free of smudges or fingerprints.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowBrowse More Related Video

UV Vis spectroscopy explained lecture

Chemistry Class 12 | Chapter 12 | Topic 3b | UV-VIS Spectroscopy | in urdu | tutoria.pk

Spektrofotometer UV Vis Double Beam - Pengertian, Fungsi, Jenis-Jenis dan Bagian-Bagian
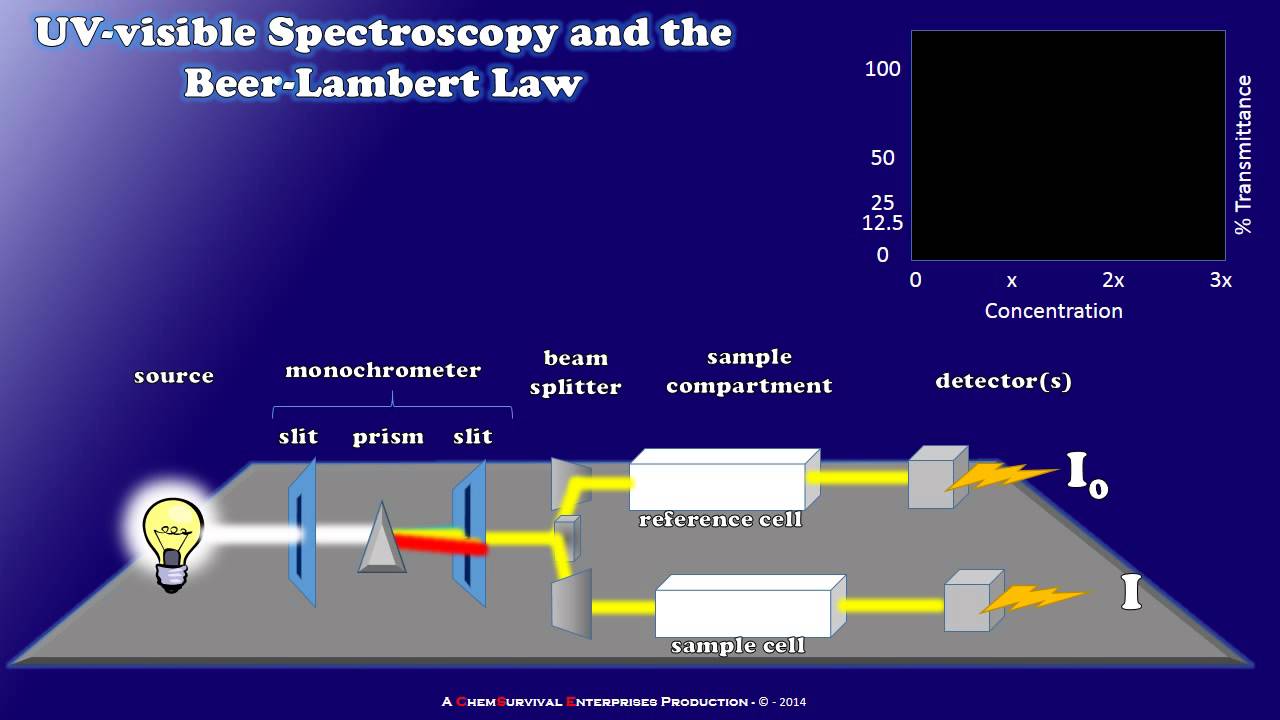
How a Simple UV-visible Spectrophotometer Works

SPEKTROFOTOMETRI UV-Vis | Prinsip Kerja Spektrofotometri UV-Vis
UV-visible spectroscopy basics- Chromophore, auxochrome, spectral shifts
5.0 / 5 (0 votes)