Levels of Protein Structure
Summary
TLDRThis video explains the four levels of protein structure—primary, secondary, tertiary, and quaternary—and their importance in protein function. It highlights the role of weak interactions in stabilizing these structures and differentiates between secondary structures like alpha helixes and beta sheets. The video also demonstrates how changes in pH can denature proteins by disrupting these interactions, while chaperonins help proteins fold correctly in cells. It emphasizes that the sequence of amino acids (primary structure) determines the final folded form and function of a protein.
Takeaways
- 😀 The primary structure of a protein is the sequence of amino acids connected by peptide bonds.
- 🔍 Secondary structure considers hydrogen bonding between the backbone atoms, leading to specific folding patterns like alpha-helices and beta-pleated sheets.
- 🧬 Tertiary structure involves the folding of the protein chain based on weak interactions such as hydrogen bonds, ionic bonds, and hydrophobic interactions involving the R groups of amino acids.
- 🌐 Quaternary structure pertains to proteins composed of multiple polypeptide chains that interact through weak interactions to form a functional complex.
- 🔗 The function of a protein is determined by its three-dimensional structure, which is a result of the folding dictated by its primary structure.
- 🌡️ Changes in pH can disrupt the weak interactions within a protein, leading to denaturation and loss of function, but this can be reversible if conditions return to optimal levels.
- 🔬 The video emphasizes the importance of understanding the relationship between pH, protonation, and deprotonation of amino acid side chains in protein folding and function.
- 🌟 Chaperonins are proteins that assist in the correct folding of other proteins by providing an isolated environment, preventing them from becoming tangled with other cellular proteins.
- 🌿 Proteins evolve to function optimally under specific conditions, including pH, temperature, and ionic strength, and deviations from these can lead to denaturation.
- 📚 The script is designed to reinforce the understanding of protein structures and their importance through clear demonstrations and the connection to previously learned concepts.
Q & A
What is the primary structure of a protein?
-The primary structure of a protein is the specific sequence of amino acids connected by peptide bonds in a polypeptide chain.
How does the secondary structure of proteins form?
-The secondary structure forms through hydrogen bonds between electronegative oxygen atoms and partially positively charged hydrogen atoms in the polypeptide backbone, creating alpha helices and beta pleated sheets.
What is the difference between an alpha helix and a beta pleated sheet?
-An alpha helix forms when the polypeptide chain twists around a central axis, creating hydrogen bonds between amino acids four residues apart. A beta pleated sheet forms when the polypeptide chain folds back on itself or runs parallel with another chain, allowing hydrogen bonds to form across the chains.
What role do R groups play in the tertiary structure of proteins?
-R groups (side chains) play a key role in the tertiary structure by forming interactions such as hydrogen bonds, ionic bonds, hydrophobic interactions, and disulfide bridges, causing the protein to fold in a specific way.
What is quaternary protein structure?
-Quaternary protein structure refers to the assembly of multiple polypeptide chains into a larger protein complex. The interactions between these chains are typically weak interactions rather than covalent bonds.
Why is the proper folding of a protein important?
-Proper folding is crucial because the specific three-dimensional structure of a protein determines its function. If a protein is misfolded, it can lose its functional properties.
How does pH affect protein structure?
-Changes in pH can disrupt ionic interactions within a protein by altering the protonation states of amino acids, leading to denaturation, where the protein unfolds and loses its functional structure.
What is the role of chaperonins in protein folding?
-Chaperonins provide an isolated environment for proteins to fold correctly, preventing them from getting tangled with other proteins during the folding process.
What happens to a protein when it is placed in non-optimal environmental conditions?
-In non-optimal conditions (e.g., extreme pH or temperature), weak interactions that maintain the protein's structure can break, causing the protein to denature and lose its function.
Can a denatured protein regain its structure and function?
-Yes, if a denatured protein is returned to optimal conditions, it can renature and regain its proper structure and function, provided no permanent damage has occurred.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowBrowse More Related Video
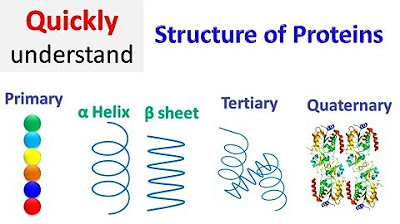
Protein structure | Primary | Secondary | Tertiary | Quaternary

Praktikum Biokima Farmasi : Analisis Struktur Protein

Proteínas | Compostos Orgânicos | Bioquímica | Prof. Paulo Jubilut
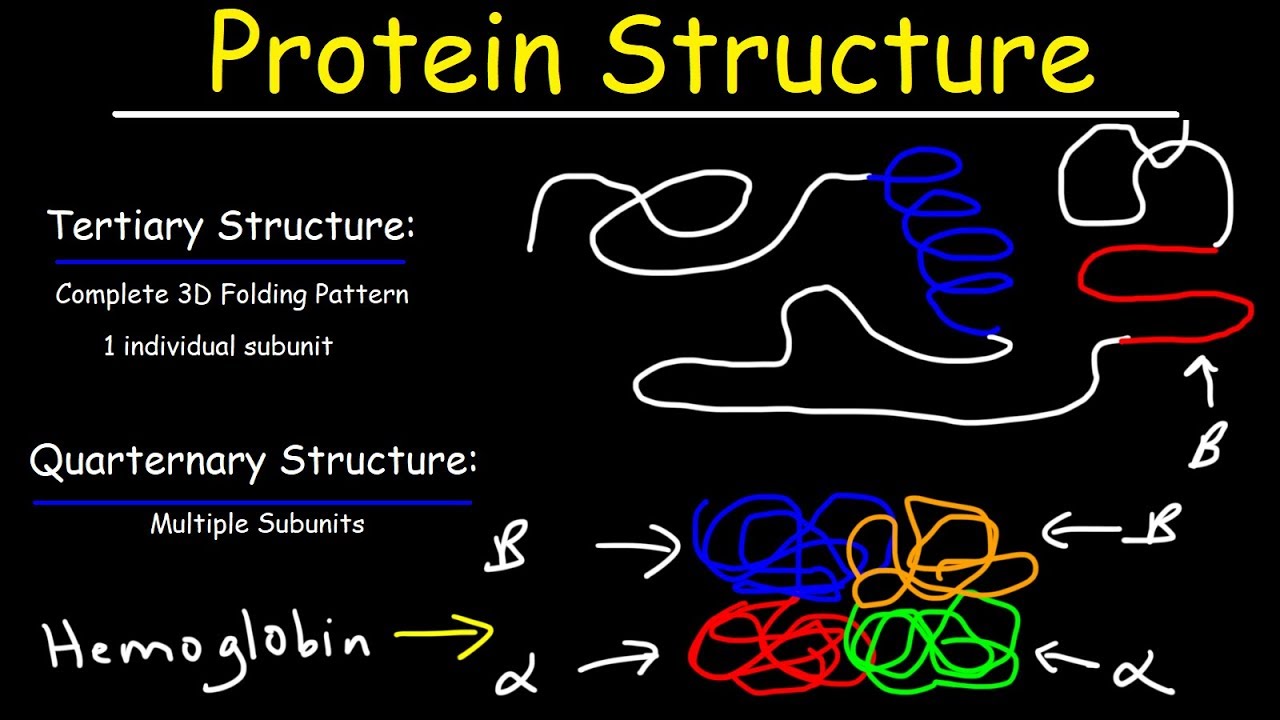
Protein Structure - Primary, Secondary, Tertiary, & Quarternary - Biology

Video11 Ch 3 Act 1part 2
The Central Dogma: DNA to proteins (an animated lecture video)
5.0 / 5 (0 votes)