Praktikum Biokima Farmasi : Analisis Struktur Protein
Summary
TLDRThis video script delves into the intricate world of protein structure analysis in biochemistry labs. It explains the classification of proteins based on the number of amino acids, the four levels of protein structure (primary, secondary, tertiary, and quaternary), and the impact of a single amino acid change on diseases like sickle cell anemia. The script also covers the importance of hydrogen bonds in secondary structure and introduces the use of informatics in protein analysis, including databases like MCPE and Sanger. The practical session involves analyzing a specific protein, identifying its structure levels, and exploring its isoelectric point and scientific articles related to its function and diseases.
Takeaways
- 😀 The video script discusses the final topic of the biochemistry lab, which is the structure analysis of proteins.
- 🔍 Proteins are composed of amino acid chains and are classified based on the number of amino acids present in the chain, such as peptides, dipeptides, tripeptides, and polypeptides.
- 🧬 Proteins generally consist of 100-10,000 amino acids linked together and are the building blocks of life.
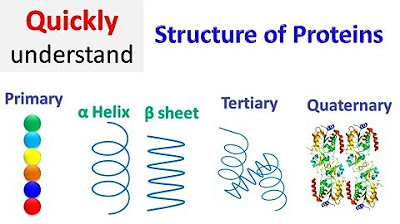
- 🌐 There are four levels of protein structure: primary (linear sequence of amino acids), secondary (shape based on peptide chain folding like alpha helix), tertiary (3D shape of the folded polypeptide chain), and quaternary (arrangement of multiple polypeptide chains).
- 🔬 The script highlights the importance of the primary structure, where even a single amino acid change can significantly affect the protein's function and lead to diseases like sickle cell anemia.
- 🔑 Secondary structures are stabilized by hydrogen bonds, with alpha helix and beta sheets being common examples.
- 🤝 Tertiary structure involves various interactions such as hydrogen bonds, ionic bonds, disulfide bonds, and hydrophobic interactions that give the protein its 3D shape.
- 🌟 Quaternary structure refers to proteins made up of multiple polypeptide chains or subunits that together perform a specific function.
- 📚 The script introduces the use of bioinformatics to analyze protein structures using molecular information related to DNA, RNA, and amino acids, facilitated by software and databases.
- 💻 The lab session involves working with a specific protein code, using methodologies outlined in lab manuals and video tutorials, and analyzing the protein's structure and isoelectric point.
- 📝 Participants are expected to document their findings, explain the significance of the protein's structure, and research its relation to diseases or other targets.
Q & A
What is the basic unit of a protein?
-The basic unit of a protein is the amino acid, which is composed of carbon, hydrogen, oxygen, and nitrogen.
How are proteins classified based on the number of amino acids?
-Proteins are classified based on the number of amino acids they contain: peptides with less than 50 amino acids, dipeptides with two amino acids, tripeptides with three amino acids, and polypeptides with more than 10 amino acids. Proteins generally consist of 100-10,000 amino acids.
What are the four levels of protein structure?
-The four levels of protein structure are primary (linear sequence of amino acids), secondary (local folding patterns like alpha-helix and beta-sheet), tertiary (three-dimensional structure of the polypeptide chain), and quaternary (arrangement of multiple polypeptide chains).
What is the significance of the primary structure of a protein?
-The primary structure, which is the sequence of amino acids, is crucial as even a single amino acid change can significantly alter the protein's secondary, tertiary, and quaternary structures, affecting its function.
How does a change in a single amino acid affect a protein?
-A change in a single amino acid can alter the protein's conformation, potentially disrupting its secondary and tertiary structures, and consequently, its function, as seen in diseases like sickle cell anemia.
What stabilizes the secondary structure of proteins?
-The secondary structure of proteins is stabilized by hydrogen bonds, which form between the backbone atoms of the polypeptide chain.
What are the types of interactions that contribute to the tertiary structure of a protein?
-The tertiary structure of a protein is stabilized by various interactions including hydrogen bonds, ionic bonds, disulfide bridges, and hydrophobic interactions.
What is the quaternary structure of a protein?
-The quaternary structure refers to the arrangement of multiple polypeptide chains or subunits, which together form a functional multi-subunit protein complex.
Why is the study of protein structure important in understanding diseases?
-Studying protein structure is important in understanding diseases because abnormalities in protein structure can lead to various conditions, such as sickle cell anemia, where a single amino acid change in hemoglobin alters its shape and function.
How can computational approaches aid in protein structure analysis?
-Computational approaches, such as bioinformatics, involve the use of software and databases to analyze molecular data related to DNA, RNA, and proteins, facilitating the understanding of protein structure and function.
What is the purpose of the practical exercise mentioned in the script?
-The practical exercise aims to explore protein structures using computational methods, where students will analyze a specific protein code, determine its secondary and tertiary structures, and understand its isoelectric point using databases like ExPASy.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowBrowse More Related Video

Protein Isolation (Electrophoresis, Isoelectric Focusing, Chromatography) & Protein Analysis 🧐 🧪

Introduction to Biochemistry
Protein structure | Primary | Secondary | Tertiary | Quaternary

Proteínas, aminoácidos e desnaturação - Aula 6 - Mód. 1 - Bioquímica e Biologia Celular | Prof. Gui

The Map of Chemistry

cAMP
5.0 / 5 (0 votes)