51. Hydrogen Spectrum | Chapter 12 Atom | Physics Baba 2.0
Summary
TLDRThis detailed video script delves into the hydrogen spectrum, explaining how electron transitions between energy levels create various spectral series like Lyman, Balmer, and others. The teacher explains these concepts with relatable examples, using analogies like a cashier being promoted to a bank manager to simplify complex ideas. Key topics include energy calculations for electron transitions, the significance of spectral lines, and the application of formulas such as the Rydberg constant. The script combines scientific rigor with engaging storytelling to make the topic of atomic spectra more accessible and memorable.
Takeaways
- 😀 The spectrum of light is formed when electrons absorb energy and jump to higher orbits, then emit energy as they return to lower orbits.
- 😀 The hydrogen spectrum is divided into different series like the Lyman, Balmer, and Paschen series based on the energy levels the electron transitions between.
- 😀 The Lyman series corresponds to ultraviolet light, the Balmer series to visible light, and the Paschen series to infrared light.
- 😀 Energy differences between electron orbits are crucial for determining the wavelength and type of radiation emitted (UV, visible, or IR).
- 😀 The energy required to move an electron to a higher orbit is the difference between the energy levels, which can be calculated using the formula E = -31.6/n^2.
- 😀 For the Lyman series, when electrons jump to the first orbit (n=1), they emit energy in the UV range, with higher energy transitions producing shorter wavelengths.
- 😀 The Balmer series, when electrons return to the second orbit (n=2), produces visible light, with different lines corresponding to different wavelengths.
- 😀 The Paschen series results from electrons returning to the third orbit (n=3), which emits infrared light, with longer wavelengths than visible light.
- 😀 A critical concept in the script is the inverse relationship between the energy of the electron and the distance from the nucleus (higher energy means closer orbit).
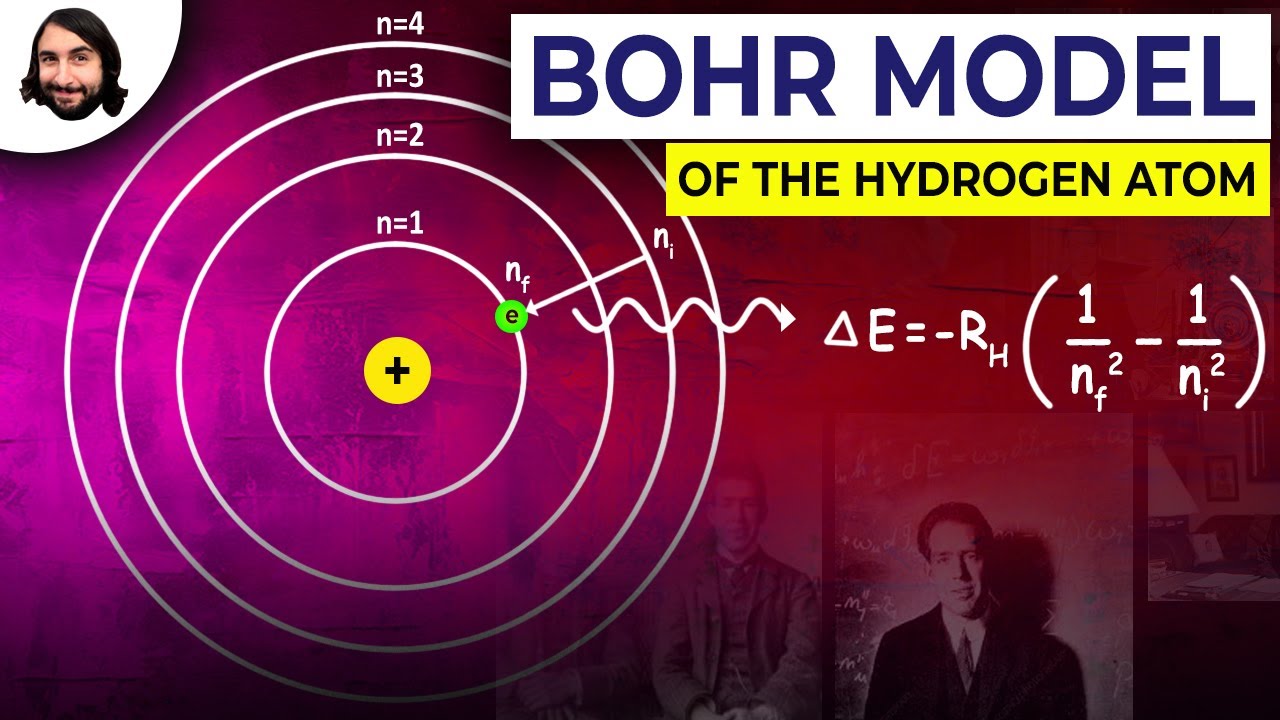
- 😀 The formula for energy transitions between levels, ΔE = -13.6 eV * (1/n1^2 - 1/n2^2), allows us to calculate the energy of emitted or absorbed radiation during electron transitions.
Q & A
What is the hydrogen spectrum and how is it formed?
-The hydrogen spectrum is formed when an electron in a hydrogen atom absorbs energy and jumps to a higher orbit, then releases energy as it returns to a lower orbit. This energy release produces specific wavelengths of light, forming the hydrogen spectrum.
What happens when an electron in a hydrogen atom moves from a lower to a higher orbit?
-When an electron in a hydrogen atom moves from a lower to a higher orbit, it absorbs energy. The energy difference between the orbits is specific, and this absorbed energy corresponds to a particular wavelength of light.
What is the concept of energy levels in atomic structure?
-In atomic structure, energy levels refer to the fixed orbits where electrons are found. These levels are quantized, meaning electrons can only occupy specific energy states. The energy difference between these levels determines the wavelengths of light emitted or absorbed.
What are the Lyman, Balmer, and other series in the hydrogen spectrum?
-The Lyman series corresponds to transitions where the electron moves to the n=1 level, emitting ultraviolet light. The Balmer series corresponds to transitions to the n=2 level, emitting visible light. Other series like the Paschen, Brackett, and Pfund correspond to transitions to higher levels, emitting infrared light.
What is the significance of the energy difference between levels in hydrogen atoms?
-The energy difference between electron orbits in a hydrogen atom determines the wavelength of the emitted or absorbed light when the electron moves between these levels. Larger energy differences correspond to shorter wavelengths (higher energy), while smaller differences correspond to longer wavelengths (lower energy).
How do energy levels and wavelengths relate in the hydrogen atom?
-Energy levels in the hydrogen atom are directly related to the wavelengths of light emitted or absorbed during electron transitions. The difference in energy between two levels determines the wavelength of the radiation according to the formula: E = hc/λ, where E is energy, h is Planck's constant, c is the speed of light, and λ is wavelength.
What happens when an electron jumps from the n=1 orbit to the n=2 orbit?
-When an electron jumps from the n=1 orbit to the n=2 orbit, it absorbs energy. The energy required for this transition corresponds to the difference between the two energy levels.
Why is the concept of 'quantization' important in understanding atomic spectra?
-Quantization is important because it explains why only certain wavelengths of light are emitted or absorbed by atoms. Since electrons can only exist in specific energy levels, the energy differences between these levels lead to distinct spectral lines, forming the atomic spectrum.
How do the formulas for energy levels help in calculating the energy required for transitions?
-The formulas for energy levels, such as E = -13.6 eV/n² for hydrogen, allow us to calculate the energy of electrons at specific orbits. By finding the energy difference between two levels, we can determine the energy required for an electron to transition between them.
What are some challenges in using atomic spectra to determine the properties of atoms?
-Challenges in using atomic spectra include accounting for various series and understanding the complex interactions between energy levels, as well as the need to precisely measure the wavelengths of emitted light. Small variations in energy calculations or external factors can complicate the process.
Outlines

Этот раздел доступен только подписчикам платных тарифов. Пожалуйста, перейдите на платный тариф для доступа.
Перейти на платный тарифMindmap

Этот раздел доступен только подписчикам платных тарифов. Пожалуйста, перейдите на платный тариф для доступа.
Перейти на платный тарифKeywords

Этот раздел доступен только подписчикам платных тарифов. Пожалуйста, перейдите на платный тариф для доступа.
Перейти на платный тарифHighlights

Этот раздел доступен только подписчикам платных тарифов. Пожалуйста, перейдите на платный тариф для доступа.
Перейти на платный тарифTranscripts

Этот раздел доступен только подписчикам платных тарифов. Пожалуйста, перейдите на платный тариф для доступа.
Перейти на платный тарифПосмотреть больше похожих видео
5.0 / 5 (0 votes)