PERSAMAAN REAKSI KIMIA (Kelas 10)
Summary
TLDRIn this educational video, viewers learn about the process of balancing chemical reactions. The video covers the definition of chemical reactions, their characteristics, and the law of conservation of mass. It introduces four phases of matter and key terms like reactants, products, coefficients, and indices. The video explains two methods for balancing chemical reactions: the simple method (balancing cations, anions, hydrogen, and oxygen) and the algebraic method, which is used for more complex reactions. This tutorial makes the concept of balancing chemical equations easy to understand for students, offering clear examples and practical tips.
Takeaways
- 😀 A chemical reaction is a permanent change where substances transform into new ones, like iron rusting.
- 😀 Key signs of a chemical reaction include the formation of a precipitate, gas, color change, and temperature change.
- 😀 The Law of Conservation of Mass states that mass and the number of atoms remain constant before and after a chemical reaction.
- 😀 Four phases of matter in chemical reactions are solid (s), liquid (l), aqueous (aq), and gas (g).
- 😀 Reactants are the substances before the reaction, while products are the substances formed afterward.
- 😀 Coefficients represent the number of molecules or atoms involved in the reaction, while subscripts show the number of atoms in a molecule.
- 😀 Balancing chemical reactions involves ensuring that the number of atoms on both sides of the equation is the same.
- 😀 The order of balancing atoms is to first balance cations, then anions, followed by hydrogen, and finally oxygen.
- 😀 The algebraic method for balancing reactions is used for more complex reactions and involves assigning variables to compounds.
- 😀 To balance reactions algebraically, create equations for each element involved and solve them systematically.
- 😀 Practicing balancing reactions makes it easier to ensure that the number of atoms is the same on both sides of the equation.
Q & A
What is a chemical reaction?
-A chemical reaction is a process where substances change into new substances that cannot easily be reversed, such as the rusting of iron. These reactions result in the formation of new substances.
What are the four characteristics that indicate a chemical reaction has occurred?
-The four characteristics are: 1) Formation of a precipitate, 2) Formation of gas, 3) Change in color, and 4) Change in temperature.
What is the principle behind balancing chemical reactions?
-The principle behind balancing chemical reactions is based on the Law of Conservation of Mass, which states that the mass of the substances before and after the reaction remains constant. Therefore, the number of atoms of each element must be the same on both sides of the reaction.
What are the four phases (states) of matter in a chemical reaction?
-The four phases are: 1) Solid (S), 2) Liquid (L), 3) Aqueous (aq), and 4) Gas (G).
What do the terms 'reactant' and 'product' mean in a chemical reaction?
-The 'reactant' refers to the substances that participate in the chemical reaction, while the 'product' refers to the new substances that are formed as a result of the reaction.
What is the difference between coefficient and index in a chemical equation?
-The coefficient represents the number of molecules or atoms involved in the reaction, while the index refers to the number of atoms of an element in a single molecule of a compound.
How is a chemical equation balanced using the Kaho method?
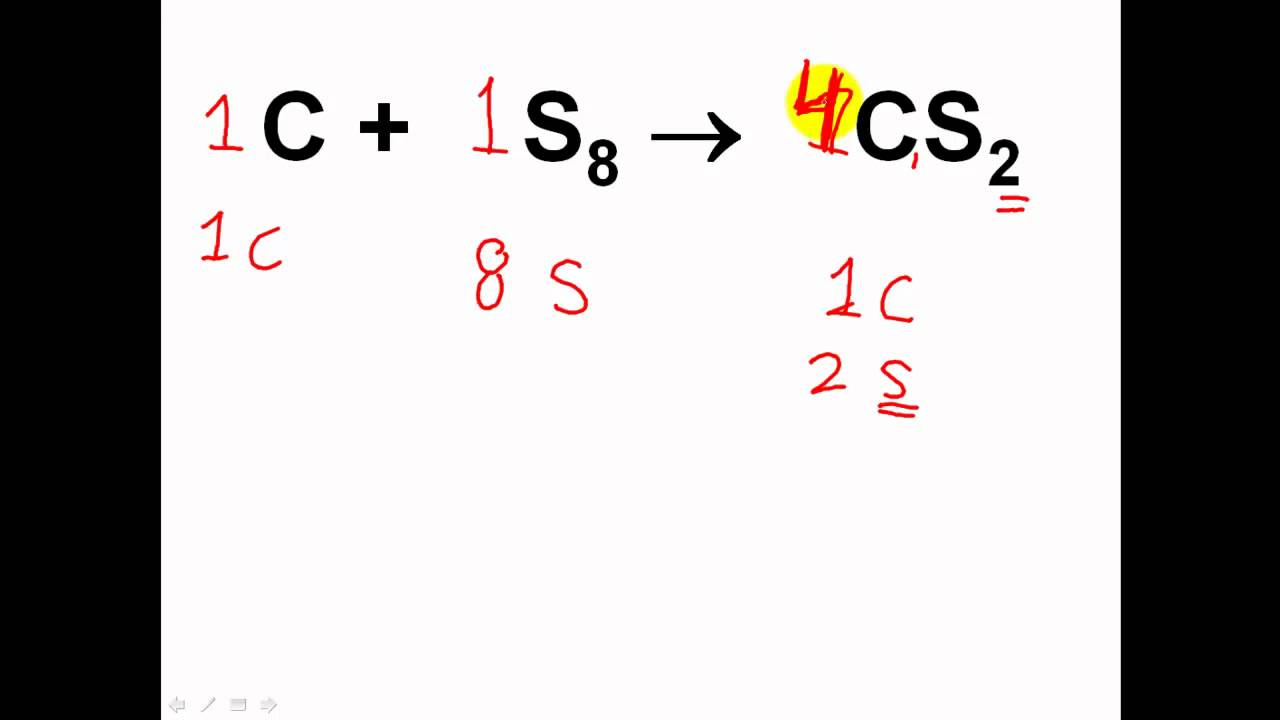
-In the Kaho method, the balancing process is carried out step by step, starting with balancing the cations, then anions, followed by hydrogen and oxygen atoms. This method ensures that the number of atoms on both sides of the equation is equal.
What does it mean to balance a chemical equation algebraically?
-Balancing a chemical equation algebraically involves assigning variables to the coefficients of each compound in the reaction. Then, using algebraic equations, you solve for the coefficients that balance the atoms of each element on both sides of the equation.
Why do we multiply coefficients to eliminate fractions in a balanced chemical equation?
-Multiplying coefficients helps to eliminate fractions and make the coefficients whole numbers. This step is necessary to present the balanced equation in a simpler, more understandable form.
What are the steps involved in balancing a complex chemical equation using algebra?
-The steps include identifying all the elements involved, assigning variables to each compound, forming equations based on the number of atoms on both sides, and then solving these equations to find the correct coefficients for each substance.
Outlines

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレードMindmap

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレードKeywords

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレードHighlights

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレードTranscripts

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレード関連動画をさらに表示

Persamaan reaksi dan penyetaraan reaksi kimia - Kimia SMA kelas 10 semester 2

Balancing Chemical Equations

GCSE Chemistry Revision "Balancing Chemical Equations"

Manufacturing Process of Propylene Oxide
How to Balance Chemical Equations & Reactions 1 - EASY!

Percobaan Elektrolisis KI | Lab Kimia | UPT Laboratorium UHO
5.0 / 5 (0 votes)
