GCSE Chemistry Revision "Balancing Chemical Equations"
Summary
TLDRThis educational video from Free Science Lessons guides viewers through the process of balancing chemical equations, a topic often perceived as challenging. It begins by explaining the components of chemical formulas, using sodium carbonate as an example. The video emphasizes the importance of maintaining the integrity of small numbers in formulas while using large numbers to balance equations. Through step-by-step examples, including calcium and chlorine reactions, viewers learn to identify and correct imbalances. The instructor encourages practice with sample equations, ultimately equipping students with the skills needed to balance equations confidently.
Takeaways
- 😀 A chemical formula represents the composition of a compound using symbols for elements.
- 🧪 The small numbers in a chemical formula indicate the number of atoms of each element present.
- ❗ Changing small numbers in a chemical formula results in a different molecule and is not allowed.
- 🔢 Large numbers placed in front of a chemical formula indicate how many molecules are present.
- ⚖️ A balanced chemical equation has the same number of atoms of each element on both sides of the equation.
- 🔍 To balance an equation, first count the number of atoms for each element on both sides.
- 📈 If an element's atoms are unbalanced, use large numbers to adjust the count on the appropriate side.
- 💡 You typically will not be asked to balance an entire equation in an exam but rather a specific part.
- ✏️ Practice examples are essential for mastering the skill of balancing chemical equations.
- 📚 Additional resources, like revision workbooks, can provide more practice questions on this topic.
Q & A
What is a chemical formula?
-A chemical formula represents the composition of a compound, indicating the elements present and the number of atoms of each element.
Why are capital letters important in chemical formulas?
-Capital letters denote the different elements in a chemical formula, helping to identify which elements are present in the compound.
How do you interpret subscripts in a chemical formula?
-Subscripts indicate the number of atoms of the element in the compound. For example, in Na₂CO₃, the subscript '2' indicates there are two sodium atoms.
What is the rule regarding changing subscripts in a chemical formula?
-You are never allowed to change the subscripts in a chemical formula, as doing so would create a different molecule.
What does placing a large number in front of a chemical formula signify?
-A large number in front of a chemical formula indicates the number of molecules of that compound, affecting the overall count of atoms in the reaction.
How can you tell if a chemical equation is balanced?
-A chemical equation is balanced if the number of atoms of each element on the reactants side equals the number on the products side.
In the equation Na + I₂ → NaI, what adjustment is needed to balance it?
-To balance the equation, you would place a coefficient of 2 in front of NaI, making it Na + I₂ → 2NaI, which balances the sodium and iodine atoms.
What adjustments are made in the reaction of calcium oxide with hydrochloric acid?
-In the reaction, placing a coefficient of 2 in front of hydrochloric acid (HCl) balances the hydrogen and chlorine atoms, resulting in CaO + 2HCl → CaCl₂ + H₂O.
What is the first step to take when balancing a chemical equation?
-The first step is to count the number of atoms of each element on both sides of the equation to determine which are unbalanced.
How can you practice balancing chemical equations effectively?
-You can practice by trying various examples, starting with simpler equations and gradually working up to more complex ones, often found in revision workbooks or educational resources.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowBrowse More Related Video

Introduction to Balancing Chemical Equations
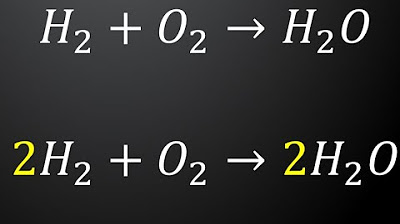
Chemistry: Balancing Chemical Equations (Tagalog Explained)
Balancing Chemical Equations

Persamaan reaksi dan penyetaraan reaksi kimia - Kimia SMA kelas 10 semester 2

Physical Science : 5 EASY STEPS in BALANCING EQUATIONS (TAGALOG) with Explanation and Full Examples

REACCIONES ORGANICAS DE COMBUSTIÓN | Ejercicios con Alcanos
5.0 / 5 (0 votes)