Physics 35 Coulomb's Law (1 of 8)
Summary
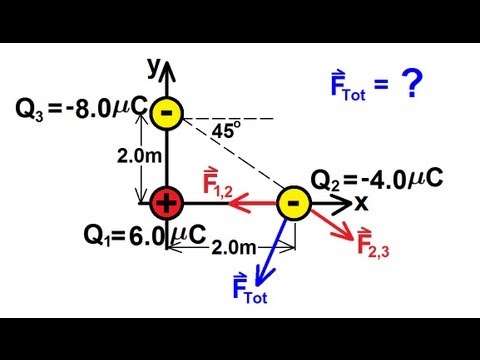
TLDRIn this physics tutorial, the concept of Coulomb's law is explored through a problem involving two charges: a 6-microcoulomb charge at the origin and a -4-microcoulomb charge placed 2 meters to the right. The forces on each charge due to the other are calculated using Coulomb's law, and the magnitude and direction of these forces are derived. The problem emphasizes the attraction between oppositely charged particles, with the magnitude of the force on both charges being 0.054 N. The tutorial also hints at more complex problems involving charges not aligned in a straight line.
Takeaways
- 😀 Coulomb's Law describes the force between two charges based on their magnitudes and the distance between them.
- 😀 The equation for Coulomb's force is F = k * |q1 * q2| / r^2, where k is a constant (9 * 10^9 N m²/C²), q1 and q2 are charges, and r is the distance.
- 😀 The problem involves two charges: one positive charge of +6.0 μC at the origin and one negative charge of -4.0 μC placed 2 meters away.
- 😀 Forces between opposite charges attract each other, as seen with the force on q1 being directed towards q2 and vice versa.
- 😀 To calculate the force, the magnitudes of the charges and the distance between them are used in Coulomb's Law.
- 😀 The force on each charge is found to be 0.054 N, indicating the attraction between the two charges.
- 😀 The force on q1 is in the positive x-direction (rightward), as it is being pulled towards the negative charge q2.
- 😀 The force on q2 is in the negative x-direction (leftward), as it is being pulled towards the positive charge q1.
- 😀 Magnitudes of forces on both charges are equal, but their directions are opposite due to the nature of the attraction between opposite charges.
- 😀 When calculating the force using Coulomb’s Law, we use the absolute values of the charges, ignoring their signs in the magnitude calculation.
Q & A
What is Coulomb's Law and what does it describe?
-Coulomb's Law describes the electrostatic force between two point charges. The force is directly proportional to the product of the charges and inversely proportional to the square of the distance between them. The equation is F = k * (q1 * q2) / r^2, where k is the Coulomb constant, q1 and q2 are the magnitudes of the charges, and r is the distance between them.
What is the value of the Coulomb constant, k?
-The Coulomb constant, k, is equal to 9 × 10^9 N·m²/C² (Newtons times square meters per coulomb squared).
What is the magnitude of the force between the two charges in the given problem?
-The magnitude of the force between the two charges is 0.054 N (Newtons). This is calculated using Coulomb's Law with the given values for the charges and the distance between them.
How do you determine the direction of the force between two charges?
-The direction of the force is determined by the nature of the charges. If the charges are of opposite signs, the force is attractive, pulling the charges toward each other. If the charges are of the same sign, the force is repulsive, pushing the charges apart.
What is the force on the first charge due to the second charge in this problem?
-The force on the first charge (q1) due to the second charge (q2) is 0.054 N in the positive X direction, as the charges are opposite in sign and therefore attract each other.
What is the force on the second charge due to the first charge in this problem?
-The force on the second charge (q2) due to the first charge (q1) is also 0.054 N, but in the negative X direction, since the charges are oppositely charged and attract each other.
Why do we only use the magnitude of the charges when calculating the force with Coulomb's Law?
-When calculating the force with Coulomb's Law, we use the magnitudes of the charges because the formula calculates the magnitude of the electrostatic force. The direction is considered separately, based on whether the charges are opposite or the same sign.
How does the distance between the charges affect the force between them?
-The electrostatic force between two charges is inversely proportional to the square of the distance between them. This means that as the distance increases, the force decreases rapidly.
Why do we express the force in terms of vectors?
-We express the force in terms of vectors because force has both magnitude and direction. Coulomb's Law gives the magnitude of the force, but the vector representation is necessary to indicate the direction of the force (toward or away from the other charge).
What challenges might arise when charges are not placed on a straight line, and how could they affect calculations?
-When charges are not placed on a straight line, the calculations become more complex because the forces between each pair of charges are not aligned along a single axis. This requires breaking the forces into components along different axes and using vector addition to find the net force.
Outlines

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレードMindmap

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレードKeywords

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレードHighlights

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレードTranscripts

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレード5.0 / 5 (0 votes)