Praktikum Kimia Projek Air Hujan
Summary
TLDRIn this educational video, the host guides viewers through a rainwater distillation experiment. The process begins with filtering rainwater using a funnel and filter paper into a beaker. After filtration, the water is heated in a distillation apparatus to separate impurities like sulfuric and nitric acids. The distillation involves observing the condensation of water vapor in a condenser, resulting in pure water collection. The experiment concludes with measuring the amount of nitric acid left behind, indicating the level of pollution in the rainwater. The video effectively demonstrates scientific techniques for water purification and pollution analysis.
Takeaways
- 🌧️ The video is about conducting a rainwater distillation experiment.
- 🔬 Required materials include stored rainwater, a set of distillation apparatus, a funnel, filter paper, and a beaker for collection.
- 🧪 Initially, rainwater is poured into a beaker for filtration.
- 📝 A filter paper is placed on top of a funnel, and the rainwater sample is poured onto it for filtration.
- 👀 The filtration process is observed until the liquid is completely filtered, and the filter paper is analyzed for residue.
- 🔄 The process can be repeated with different rainwater samples.
- 💧 After filtration, the clear liquid is transferred into a distillation flask.
- 🔥 The distillation process involves heating the rainwater to separate impurities such as sulfates and nitrates.
- 🌡️ The distillation is performed at temperatures ranging from 80 to 100 degrees Celsius to separate impurities from pure water.
- ⏳ The condensation process is observed, where water vapor from the heated rainwater condenses back into liquid form, leaving impurities behind.
- 📊 The experiment measures the amount of nitric acid left behind, which is collected in a beaker and measured to be 6.4 mL, indicating the level of pollution in the rainwater.
- 🔍 To determine the amount of sulfuric acid, a further distillation process at 100 degrees Celsius is required, where no more water comes out, indicating the end of the process.
Q & A
What is the main activity described in the script?
-The main activity described in the script is conducting a rainwater distillation experiment.
What are the required materials for the rainwater distillation experiment?
-The required materials include stored rainwater, a set of distillation equipment, a glass beaker, a funnel, filter paper, a glass for collecting the filtrate, and a beaker for collecting the distilled water.
What is the purpose of using a glass beaker in the experiment?
-The glass beaker is used to hold the rainwater sample before filtration.
What is the role of filter paper in the experiment?
-The filter paper is used to filter the rainwater sample by trapping impurities and allowing only the water to pass through.
What happens during the filtration process described in the script?
-During the filtration process, the rainwater sample is poured onto the filter paper in the funnel, and the filtration is observed until all the liquid has passed through.
What is the purpose of the distillation process in the experiment?
-The purpose of the distillation process is to separate impurities such as sulfuric acid and nitrates from the rainwater, resulting in purified water.
What is the function of the condenser in the distillation apparatus?
-The condenser in the distillation apparatus cools the vaporized water, causing it to condense back into liquid form, which is then collected separately from the impurities.
At what temperature range is the rainwater heated during the distillation process?
-The rainwater is heated at a temperature range of approximately 80 to 100 degrees Celsius to separate the nitrates and sulfuric acid from the purified water.
How is the amount of nitric acid determined in the experiment?
-The amount of nitric acid is determined by collecting the distilled water in a glass beaker and measuring the volume of the collected liquid, which in the script is found to be 6.4 mL.
What is the significance of observing the residues left in the distillation apparatus?
-Observing the residues left in the distillation apparatus helps to understand the amount and types of impurities present in the rainwater, such as sulfuric acid and nitrates.
What is the final outcome of the distillation process described in the script?
-The final outcome of the distillation process is the collection of purified water and the measurement of the impurities such as nitric acid and sulfuric acid that were separated from the rainwater.
Outlines

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレードMindmap

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレードKeywords

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレードHighlights

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレードTranscripts

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレード関連動画をさらに表示

Chem 333: Distillation with and without a vigreux column

P3 Isolasi Minyak Cengkeh dengan Distilasi Stahl
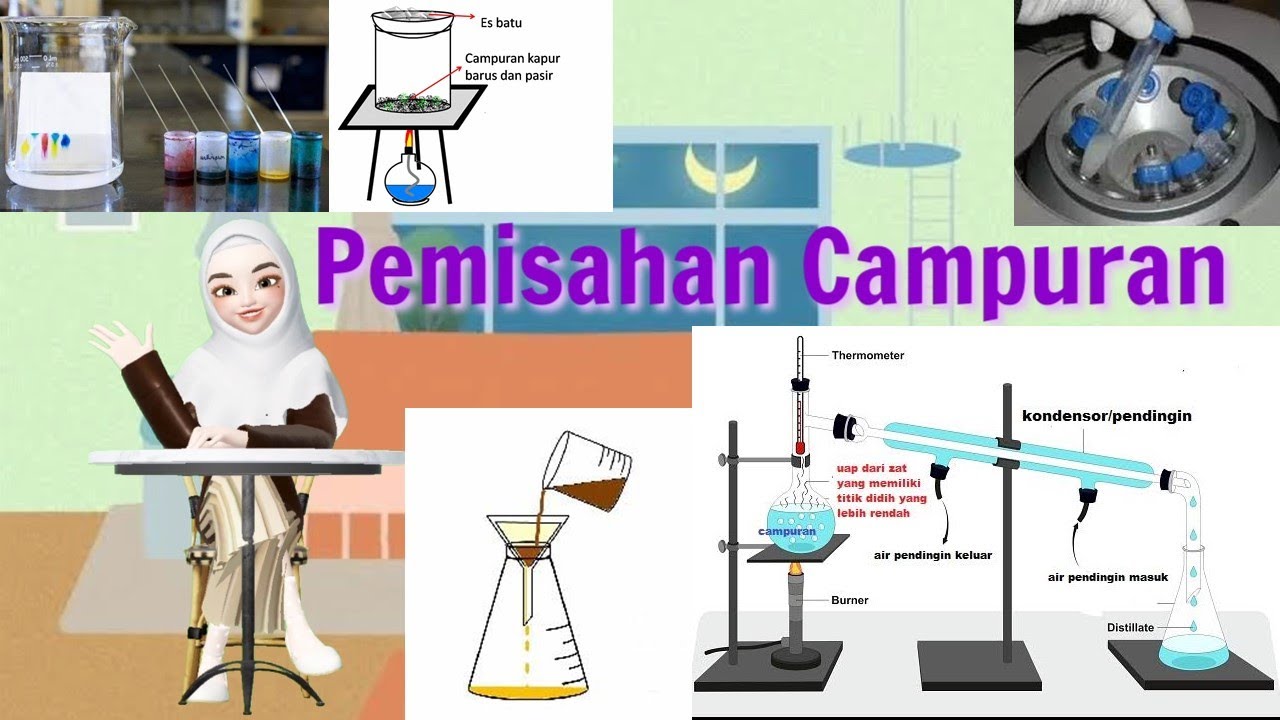
Pemisahan Campuran | Filtrasi (penyaringan), Sentrifugasi, Destilasi, Kromatografi, Sublimasi

💢SDEIN VIRTECH PRESENT💢 || Cara Merangkai Alat Praktikum Destilasi

☁️☾✧AS Level Chemistry: Paper 3 (Practical Exam) // 9701/31/O/N/20 // Question 1 - Titration♡☁️

Ecotopia - Rainwater Harvesting in Industry
5.0 / 5 (0 votes)
