Faktor-Faktor yang Mempengaruhi Pergeseran Kesetimbangan dan Penerapannya dalam Industri
Summary
TLDRThis video discusses the factors influencing chemical equilibrium, emphasizing Le Chatelier's principle. It explains how changes in concentration, volume, pressure, and temperature affect equilibrium positions. For instance, increasing reactant concentration shifts equilibrium toward products, while changes in pressure favor the side with fewer gas moles. The role of catalysts in speeding up reactions without altering equilibrium is also highlighted. Additionally, industrial applications, such as the Haber-Bosch process for ammonia production and the contact process for sulfuric acid, illustrate how these principles optimize chemical reactions for maximum yield.
Takeaways
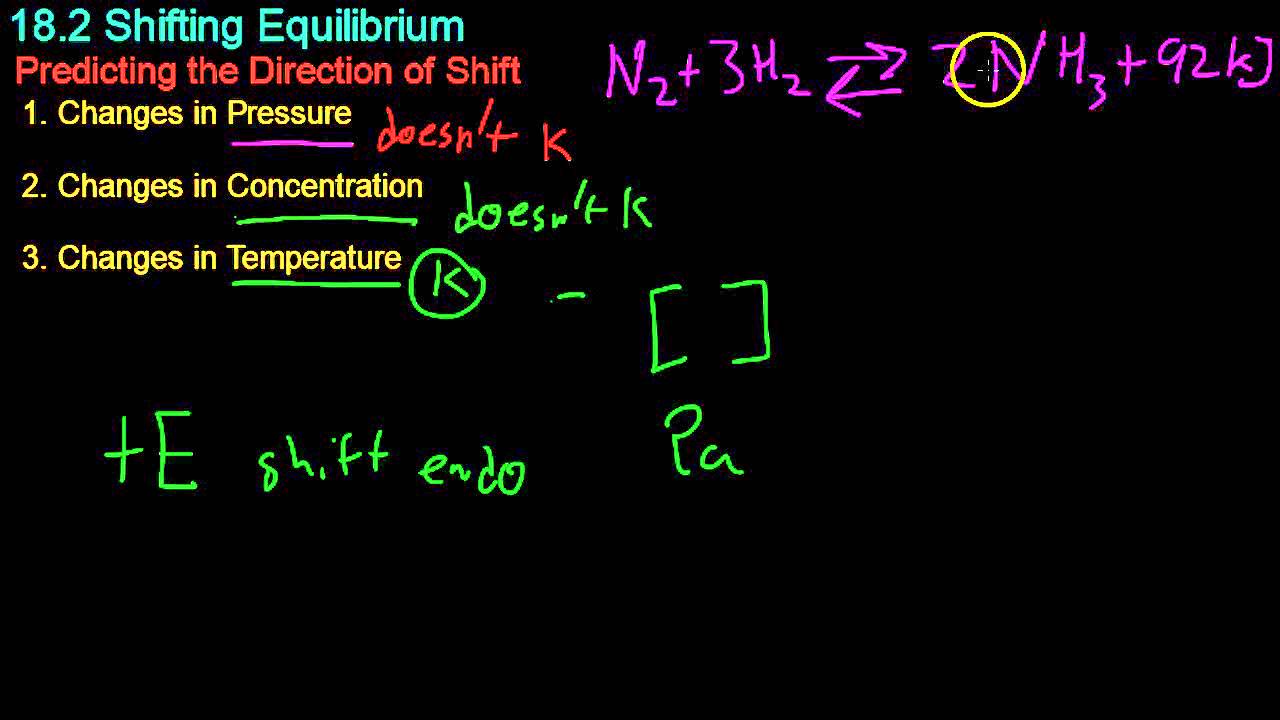
- 😀 Le Chatelier's principle states that if an external change is applied to a system at equilibrium, the system will adjust to minimize the effect of that change.
- 📈 Increasing the concentration of reactants drives the equilibrium toward the production of more products, while decreasing it shifts back toward reactants.
- 📉 Changes in pressure and volume are inversely related; increasing pressure favors the side of the reaction with fewer gas molecules.
- 🔥 Temperature changes impact exothermic and endothermic reactions differently: increasing temperature shifts exothermic reactions toward reactants and endothermic reactions toward products.
- ⏳ Catalysts accelerate the rate of reaching equilibrium but do not alter the position of the equilibrium itself.
- 🔄 The equilibrium of a reaction can be visualized as a balancing act, where changes in conditions affect the direction of the reaction.
- 💧 The equilibrium principles primarily apply to substances in solution and gases, impacting their reactions significantly.
- 🌡️ The Haber-Bosch process for ammonia production operates optimally at 500°C and high pressures, emphasizing the balance between temperature and yield.
- ⚗️ In the contact process for sulfuric acid production, the reaction is exothermic, highlighting the importance of temperature management to maintain production efficiency.
- 🔬 Understanding these principles is essential for optimizing chemical reactions in industrial applications, ensuring effective and efficient production.
Q & A
What is chemical equilibrium?
-Chemical equilibrium occurs when the rates of the forward and reverse reactions are equal, resulting in constant concentrations of reactants and products.
What does Le Chatelier's Principle state?
-Le Chatelier's Principle states that if a system at equilibrium experiences an external change, the system will adjust to counteract that change and restore equilibrium.
How does changing the concentration of reactants affect equilibrium?
-Increasing the concentration of reactants shifts the equilibrium toward products, while decreasing it shifts toward reactants.
What is the relationship between volume and pressure in the context of equilibrium?
-According to Boyle's Law, volume and pressure are inversely related. Increasing pressure (by decreasing volume) shifts equilibrium toward the side with fewer gas moles, while decreasing pressure shifts it toward the side with more gas moles.
What effect does temperature have on exothermic and endothermic reactions at equilibrium?
-For exothermic reactions, increasing temperature shifts equilibrium toward reactants, while for endothermic reactions, it shifts toward products. Conversely, decreasing temperature has the opposite effect.
How does the addition of a catalyst affect chemical equilibrium?
-A catalyst accelerates the rate of reaching equilibrium but does not change the position of equilibrium itself.
What are the optimal conditions for ammonia production using the Haber-Bosch process?
-The optimal conditions for ammonia production in the Haber-Bosch process are a temperature of 500°C and a pressure between 140 to 340 atmospheres.
What is the reaction involved in the Haber-Bosch process?
-The reaction in the Haber-Bosch process is N₂ + 3H₂ ⇌ 2NH₃, which is exothermic.
What role does continuous removal of ammonia play in the Haber-Bosch process?
-Continuous removal of ammonia shifts the equilibrium toward the right, favoring further production of ammonia.
What is the contact process for sulfuric acid production, and what conditions does it require?
-The contact process for sulfuric acid production involves the reaction 2SO₂ + O₂ ⇌ 2SO₃, which is exothermic. Optimal conditions are a temperature of 400°C and a pressure of 1 atmosphere, using vanadium(V) oxide as a catalyst.
Outlines

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレードMindmap

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレードKeywords

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレードHighlights

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレードTranscripts

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレード関連動画をさらに表示

PERGESERAN KESETIMBANGAN (ASAS LE CHATELIER) : KESETIMBANGAN KIMIA KELAS 11

Deslocamento de Equilíbrio - Princípio de Le Chatelier

11 клас. Хімія. Необоротні та оборотні хімічні реакції. Хімічна рівновага. Принцип Ле Шательє

LeChatelier Principle: Change Pressure
18.2 Shifting Equilibrium

Kimia - ANIMASI PERCOBAAN PENGARUH KONSENTRASI DAN VOLUME PADA PERGESERAN KESETIMBANGAN
5.0 / 5 (0 votes)
