Electrophilic Aromatic Substitution
Summary
TLDRIn this educational video, Professor Dave explains electrophilic aromatic substitution (EAS), a reaction where an electrophile replaces a hydrogen atom in an aromatic system like benzene. He clarifies that unlike addition reactions, EAS maintains the stability of aromaticity through a series of steps involving the formation of an arenium ion intermediate and the restoration of aromaticity by a proton extraction. The video also covers the role of Lewis acid catalysts in facilitating EAS reactions, specifically using bromination as an example. The summary concludes with the importance of understanding the mechanism and thermodynamics of EAS reactions for successful chemical synthesis.
Takeaways
- 🌟 Electrophilic Aromatic Substitution (EAS) is a reaction where an electrophile replaces a hydrogen atom in an aromatic system, unlike addition reactions which involve the interaction with a pi bond.
- 🔍 Aromatic systems are stable and prioritize maintaining their aromaticity, which is why they undergo substitution rather than addition reactions.
- 📚 The mechanism of EAS involves the formation of an arenium ion intermediate, which is a resonance-stabilized carbocation on the aromatic ring.
- ⚛️ The first step in EAS is endothermic and rate-determining because it involves the disruption of aromaticity to form the arenium ion intermediate.
- 🔄 The restoration of aromaticity in the reaction mechanism occurs when a proton is extracted from the carbon bearing the electrophile, reforming the pi bond and returning to a stable aromatic structure.
- 🧪 Specific EAS reactions, such as halogenation, require a Lewis acid catalyst to facilitate the reaction by lowering the activation energy and promoting the formation of the electrophile.
- 🌐 The use of a Lewis acid catalyst, like iron tribromide in bromination, helps to stabilize the electrophile, making it more reactive towards the aromatic ring.
- 📉 The intermediate arenium ion is at a higher energy state compared to the reactants and products, making the formation of this intermediate the most energetically unfavorable step.
- 🔗 The regeneration of the Lewis acid catalyst is crucial as it allows for the continuation of the reaction and the release of byproducts like HBr.
- 🔑 The mechanism of halogenation closely follows the generalized EAS mechanism, with the key difference being the specific electrophile and catalyst used.
- 📚 Understanding the role of the Lewis acid catalyst is essential for grasping how EAS reactions proceed and why certain conditions are necessary for the reaction to occur.
Q & A
What is the main difference between electrophilic aromatic substitution and addition reactions?
-Electrophilic aromatic substitution involves the replacement of a hydrogen atom on an aromatic ring with an electrophile, whereas addition reactions involve the interaction of a pi bond with an electrophile to form a new product. Aromatic systems prefer to maintain their stability and aromaticity, which is why they undergo substitution rather than addition.
Why is the benzene ring stable and what role does this stability play in electrophilic aromatic substitution?
-Benzene is stable due to its fully conjugated pi electrons, which form a delocalized system. This stability is crucial in electrophilic aromatic substitution because the reaction aims to maintain aromaticity by restoring the conjugated pi system after the substitution of a hydrogen atom.
What is the role of the arenium ion intermediate in the electrophilic aromatic substitution reaction?
-The arenium ion intermediate is formed when the electrophile attacks the benzene ring, leading to a positively charged intermediate where the aromaticity is temporarily lost. This step is crucial as it sets the stage for the restoration of aromaticity through the subsequent steps of the reaction.
How does the electrophilic aromatic substitution mechanism differ from an addition reaction mechanism?
-In an addition reaction, the electrophile coordinates with the cation formed after the attack on the pi bond, potentially leading to a loss of aromaticity. In contrast, in electrophilic aromatic substitution, the rest of the electrophile molecule extracts a proton from the carbon bearing the new electrophile, restoring the aromatic pi system.
Why is the first step of an electrophilic aromatic substitution reaction endothermic and rate-determining?
-The first step involves the formation of the arenium ion intermediate, which is energetically unfavorable as it disrupts the aromaticity of the benzene ring. This step is endothermic and has a high activation energy, making it the rate-determining step in the reaction.
What is the role of a Lewis acid catalyst in promoting electrophilic aromatic substitution reactions?
-A Lewis acid catalyst, such as iron tribromide in the case of bromination, lowers the activation energy of the reaction by forming a complex with the electrophile. This complex makes it easier for the benzene ring to interact with the electrophile, facilitating the electrophilic attack and subsequent substitution.
How does the presence of a Lewis acid catalyst affect the electrophilic aromatic substitution reaction?
-The Lewis acid catalyst forms a complex with the electrophile, neutralizing its formal charge and making it more reactive towards the benzene ring. This interaction lowers the activation energy of the reaction and promotes the electrophilic attack, leading to the formation of the arenium ion intermediate.
What is the significance of the delocalized positive charge and pi electron density in the arenium ion intermediate?
-The delocalized positive charge and pi electron density in the arenium ion intermediate help stabilize the intermediate, allowing it to exist as a viable species in the reaction pathway. This delocalization is crucial for the subsequent steps that restore aromaticity.
What happens in the final step of the electrophilic aromatic substitution reaction?
-In the final step, the rest of the electrophile molecule (A-) extracts a proton from the carbon bearing the new electrophile. This action restores the aromatic pi system, resulting in the formation of the substituted benzene product and the regeneration of the Lewis acid catalyst.
Can you provide an example of a specific electrophilic aromatic substitution reaction discussed in the script?
-The script discusses the bromination of benzene in the presence of a Lewis acid catalyst (iron tribromide). This reaction results in the formation of bromobenzene and HBr as a byproduct, illustrating the general mechanism of electrophilic aromatic substitution.
Outlines

Cette section est réservée aux utilisateurs payants. Améliorez votre compte pour accéder à cette section.
Améliorer maintenantMindmap

Cette section est réservée aux utilisateurs payants. Améliorez votre compte pour accéder à cette section.
Améliorer maintenantKeywords

Cette section est réservée aux utilisateurs payants. Améliorez votre compte pour accéder à cette section.
Améliorer maintenantHighlights

Cette section est réservée aux utilisateurs payants. Améliorez votre compte pour accéder à cette section.
Améliorer maintenantTranscripts

Cette section est réservée aux utilisateurs payants. Améliorez votre compte pour accéder à cette section.
Améliorer maintenantVoir Plus de Vidéos Connexes

Nucleophilic & Electrophilic Aromatic Substitution | Organic Synthesis Chemistry | Retrosynthesis
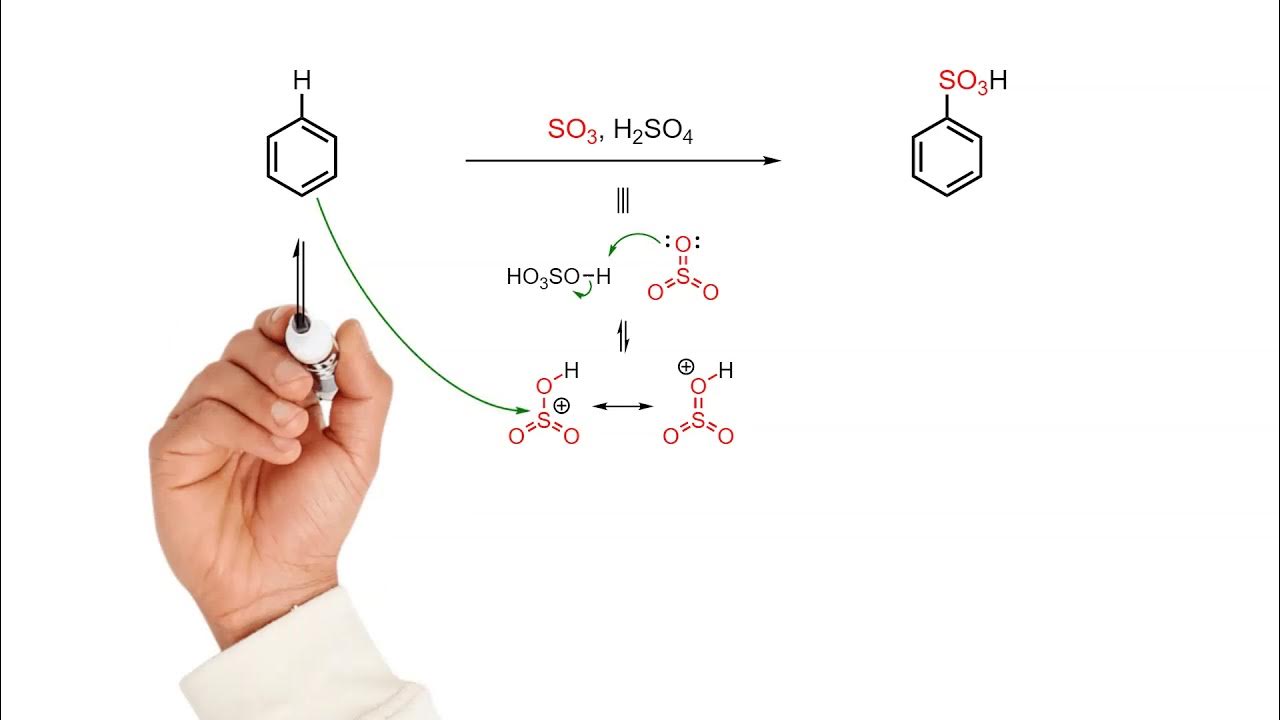
Sulfonation of benzene

Nucleophilic Aromatic Substitution

Substitusi Elektrofilik Aromatik: Sulfonasi
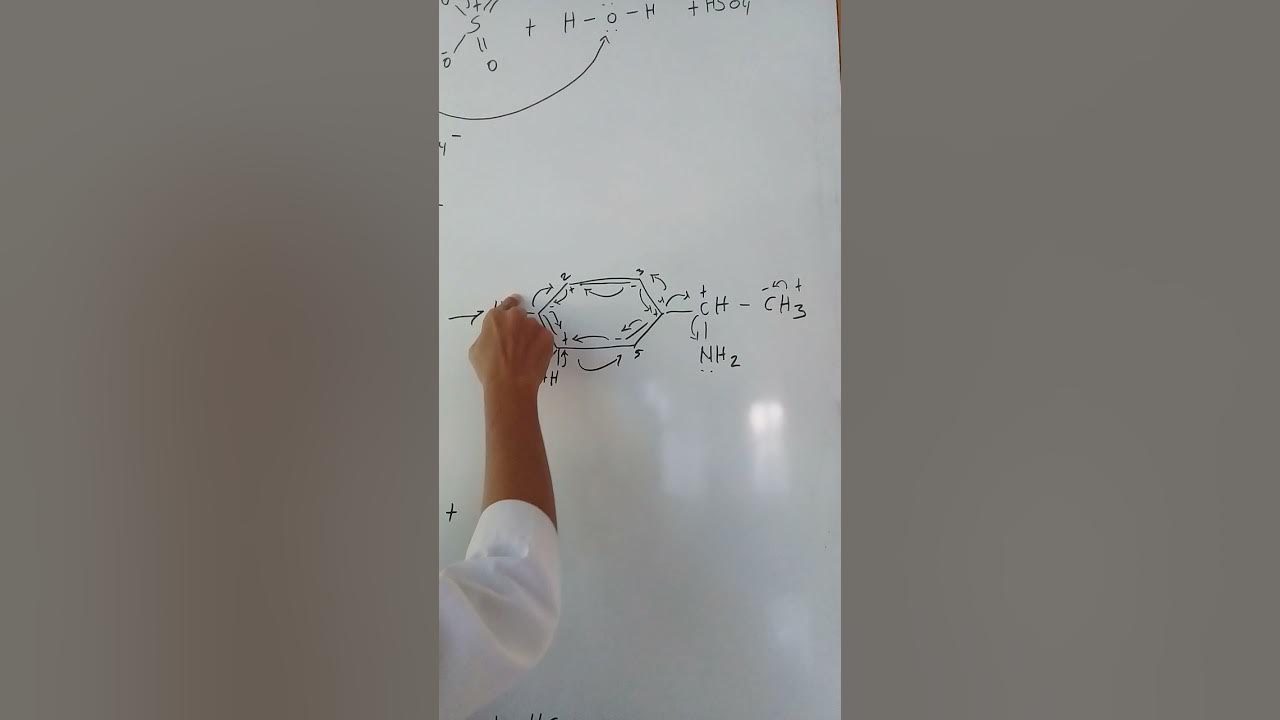
Proses Sulfonasi Senyawa Aromatik

Producción de derivados disustituidos del benceno EFECTO ORIENTADOR DE LOS DERIVADOS DEL BENCENO
5.0 / 5 (0 votes)
