How Can MASS and ENERGY be the Same Thing? What, Where and Why is it?
Summary
TLDRIn this video, the mysteries of mass and energy are explored, starting with kinetic energy and progressing to the concept of mass-energy equivalence, famously captured by E=mc^2. The video delves into how mass arises from interactions with the Higgs Field and the strong force that binds quarks inside protons and neutrons. A key insight is that most of an atom's mass comes from the energy holding quarks together, rather than the quarks' intrinsic mass. This mass-energy relationship is central to the structure of matter and has profound implications for understanding the universe's fundamental forces and particles.
Takeaways
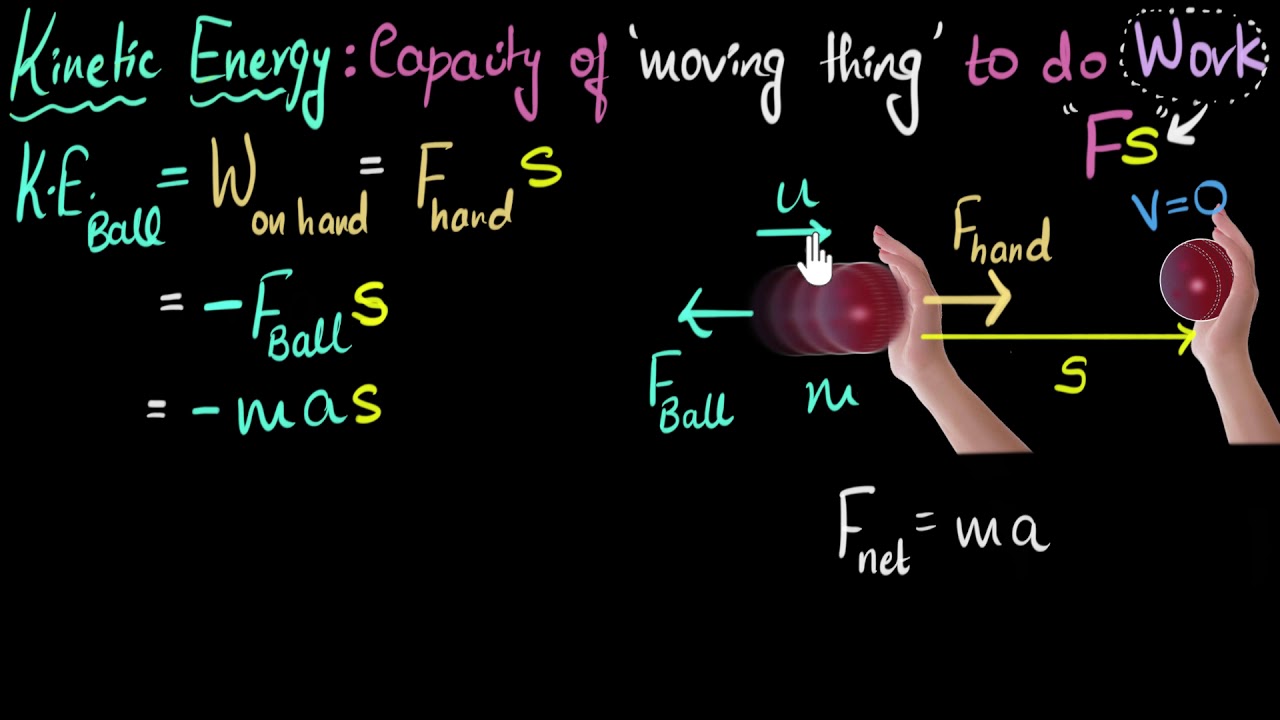
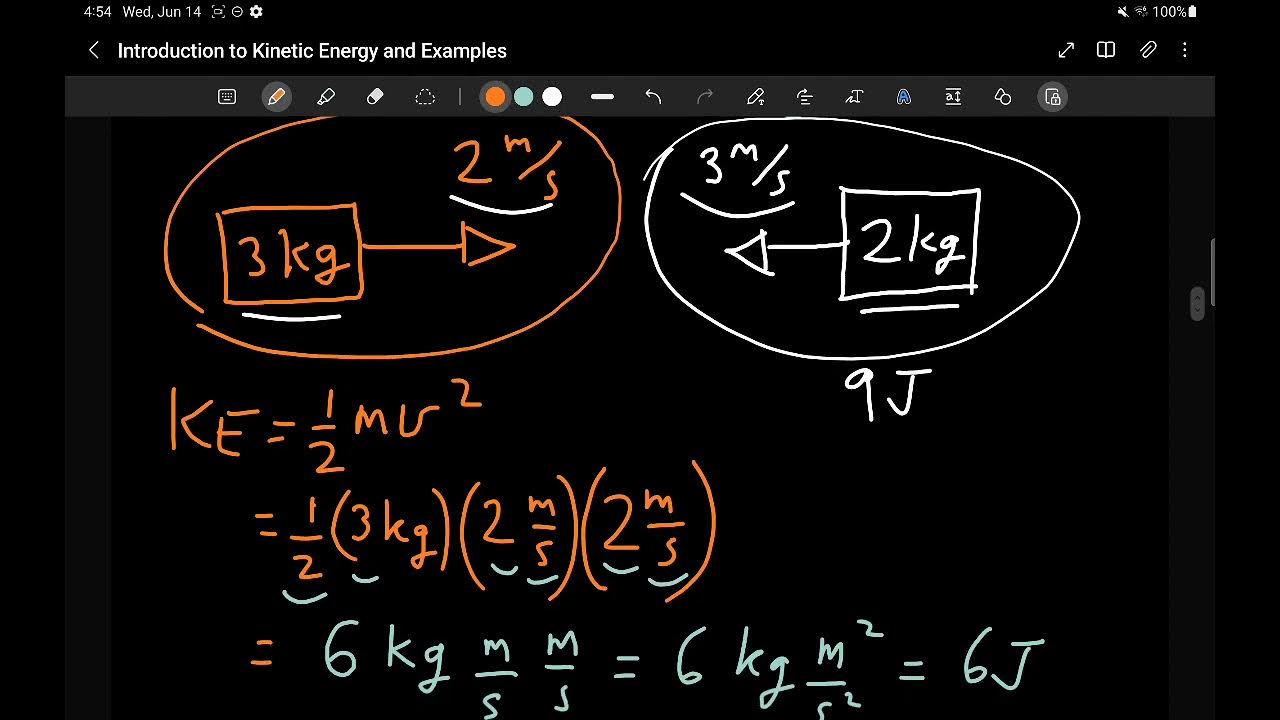
- 😀 Kinetic energy is the energy of movement, and it can be calculated using the formula ½ mv^2.
- 😀 A person standing still has much more energy than when they are moving at 100 km/h, due to mass-energy equivalence.
- 😀 Mass and energy are fundamentally related, as described by Einstein's famous equation E=mc^2.
- 😀 Most of an atom's mass is contained in the nucleus, which consists of protons and neutrons made up of quarks.
- 😀 The mass of a proton is primarily derived from the energy binding quarks together, rather than from the mass of the quarks themselves.
- 😀 The Higgs field imparts mass to fundamental particles by interacting with them, with different particles interacting with the field to varying degrees.
- 😀 Gluons, which are massless, mediate the strong force that binds quarks together inside protons and neutrons, contributing to the mass of atomic nuclei.
- 😀 The concept of 'color charge' explains how quarks interact through the strong force, which keeps them bound inside nucleons.
- 😀 The strong force is responsible for about 99% of the mass of an atom, and it can be thought of as a form of potential energy.
- 😀 Mass is essentially bound energy, and any form of energy, whether kinetic or potential, contributes to the total mass of an object.
- 😀 The strong nuclear force, which holds protons and neutrons together inside an atom's nucleus, is distinct from the strong force binding quarks within nucleons.
Q & A
What is kinetic energy and how is it calculated?
-Kinetic energy is the energy of movement. It can be calculated using the formula ½ mv^2, where 'm' is the mass of the object and 'v' is its velocity. For example, if an 80 kg person is moving at 100 km/hr, their kinetic energy would be approximately 31,000 Joules.
Why does mass have more energy than the kinetic energy of a moving object?
-Even when not moving, mass contains an incredibly large amount of energy. The energy inherent in mass is far greater than that of kinetic energy, with a stationary body having over 6*10^18 Joules of energy, due to the mass-energy equivalence principle (E=mc^2).
What is the energy in the nucleus of an atom, and where does it come from?
-The energy in the nucleus is primarily due to the strong force that binds quarks together inside protons and neutrons. This binding energy is much greater than the sum of the individual masses of the quarks themselves.
How does the Higgs field contribute to the mass of fundamental particles?
-The Higgs field interacts with fundamental particles, giving them mass. The more a particle interacts with the Higgs field, the more massive it becomes. For example, a down quark interacts more intensely with the Higgs field than an electron, resulting in a greater mass.
Why do some particles have mass while others, like photons, do not?
-Photons and gluons do not interact with the Higgs field, so they are massless. Neutrinos are a bit of a mystery, as they seem not to interact with the Higgs field, yet experiments suggest they may have a very small mass.
What is the strong force, and how does it contribute to mass?
-The strong force is a fundamental force that binds quarks together inside protons and neutrons. Although gluons, which mediate the strong force, are massless, the energy required to hold quarks together translates into mass. This energy is responsible for 99% of the mass of atomic nuclei.
What is quantum chromodynamics (QCD), and how does it explain the strong force?
-Quantum chromodynamics (QCD) is the theory that describes how the strong force works. It explains how quarks exchange 'color' charges through gluons, binding quarks together inside nucleons (protons and neutrons) and thereby contributing to the mass of an atom.
What role does the color charge play in the strong force?
-Color charge is a property of quarks and gluons that governs the strong force. Quarks constantly exchange color charges with each other, leading to the formation of neutral color combinations (red, green, and blue) within protons and neutrons. This exchange, mediated by gluons, keeps the quarks bound together.
Why can't quarks or gluons exist freely in nature?
-Quarks and gluons are confined due to a property called confinement. This means they cannot exist as free particles and are always found bound within protons, neutrons, or mesons. The strong force requires a neutral color charge combination, which is why quarks and gluons cannot exist independently.
How does the strong nuclear force differ from the strong force that binds quarks?
-The strong nuclear force, mediated by mesons, holds protons and neutrons together inside the atomic nucleus, overcoming their electromagnetic repulsion. In contrast, the strong force that binds quarks is responsible for the mass of nucleons, as it keeps the quarks within protons and neutrons tightly bound.
Outlines

Cette section est réservée aux utilisateurs payants. Améliorez votre compte pour accéder à cette section.
Améliorer maintenantMindmap

Cette section est réservée aux utilisateurs payants. Améliorez votre compte pour accéder à cette section.
Améliorer maintenantKeywords

Cette section est réservée aux utilisateurs payants. Améliorez votre compte pour accéder à cette section.
Améliorer maintenantHighlights

Cette section est réservée aux utilisateurs payants. Améliorez votre compte pour accéder à cette section.
Améliorer maintenantTranscripts

Cette section est réservée aux utilisateurs payants. Améliorez votre compte pour accéder à cette section.
Améliorer maintenant5.0 / 5 (0 votes)