4.3a Density and Composite Mixture (MJ16 P12 Q13) | AS Density | Cambridge A Level Physics
Summary
TLDRThis video explains the fundamentals of density and pressure, focusing on how to calculate and apply them in real-world scenarios. It introduces density as mass per unit volume, emphasizing its consistency across substances like gold, regardless of shape. The script then delves into the concept of composite density, showing how to calculate the density of mixtures through fractional densities or by using total mass and volume. Practical examples involving oil mixtures are provided, demonstrating how these methods are used in complex calculations. The video prepares viewers to understand the equation for pressure in fluids and the concept of buoyant force.
Takeaways
- 😀 Density is the mass per unit volume of a substance and is denoted by the Greek letter 'ρ' (rho).
- 😀 The density equation is ρ = mass/volume, with units typically in kg/m³ for standard calculations.
- 😀 Density is constant for solids and liquids, but may vary for gases due to changes in volume.
- 😀 Gases have variable density because their volume is easily compressed or expanded, affecting their density.
- 😀 For composite objects made of different materials, density can be calculated using a method called fractional density.
- 😀 When calculating the density of a mixture, you need to consider how each component contributes based on its volume and density.
- 😀 In some cases, total mass and total volume can be used to calculate the overall density of a mixture.
- 😀 Fractional density can be found by using the volume of each component and multiplying it by the respective density.
- 😀 A different method of calculating composite density is by summing up the individual masses of the components and dividing by the total volume.
- 😀 For accurate results, it's important to retain as many decimal places as possible when calculating fractional or total density.
Q & A
What is the key concept behind density in the context of this lesson?
-The key concept behind density is that it is a unique property for each substance, defined as mass per unit volume. Regardless of shape or size, materials made of the same substance will have the same density.
How do we calculate the density of an object?
-Density is calculated using the formula: ρ = m/V, where ρ is density, m is mass, and V is volume.
What is the standard unit for density in SI, and why is it important to standardize units?
-The standard unit for density in SI is kilograms per cubic meter (kg/m³). Standardizing units ensures consistency in calculations and ensures that measurements across different systems are comparable.
Why does gas not have a constant density like solids and liquids?
-Gas does not have a constant density because its volume is highly compressible. The density of a gas changes with changes in volume, unlike solids and liquids, which maintain a fixed volume.
What is composite density, and how does it differ from regular density?
-Composite density refers to the density of a mixture made up of different materials. Unlike regular density, which applies to a single substance, composite density accounts for the contribution of each component’s density in a mixture.
How do you find the density of a mixture of liquids with different densities?
-To find the density of a mixture, you can either calculate the fractional density by considering the volume contribution of each component or use the total mass of the mixture divided by the total volume.
What is the method for calculating fractional density in a mixture?
-To calculate fractional density, find the volume of each component in the mixture and multiply it by the density of that component. Then, sum these values to find the total density of the mixture.
What units are typically used in density calculations in chemistry, and how do they differ from SI units?
-In chemistry, density is often expressed in grams per cubic centimeter (g/cm³), which is more common in laboratory settings. This unit is different from the SI unit of kg/m³.
Why is it important to use total mass and total volume when calculating the density of a mixture?
-Using total mass and total volume simplifies the calculation of composite density by directly considering the entire mixture, making it easier to determine the overall density without needing to account for each component's fractional density.
How does the contribution of each component to the overall density of a mixture affect the final result?
-Each component’s contribution to the overall density is proportional to its volume in the mixture. For example, components with larger volumes will contribute more to the overall density, influencing the final result significantly.
Outlines

Cette section est réservée aux utilisateurs payants. Améliorez votre compte pour accéder à cette section.
Améliorer maintenantMindmap

Cette section est réservée aux utilisateurs payants. Améliorez votre compte pour accéder à cette section.
Améliorer maintenantKeywords

Cette section est réservée aux utilisateurs payants. Améliorez votre compte pour accéder à cette section.
Améliorer maintenantHighlights

Cette section est réservée aux utilisateurs payants. Améliorez votre compte pour accéder à cette section.
Améliorer maintenantTranscripts

Cette section est réservée aux utilisateurs payants. Améliorez votre compte pour accéder à cette section.
Améliorer maintenantVoir Plus de Vidéos Connexes
Fluida Statis • Part 1: Massa Jenis, Tekanan Hidrostatis, Gaya Hidrostatis
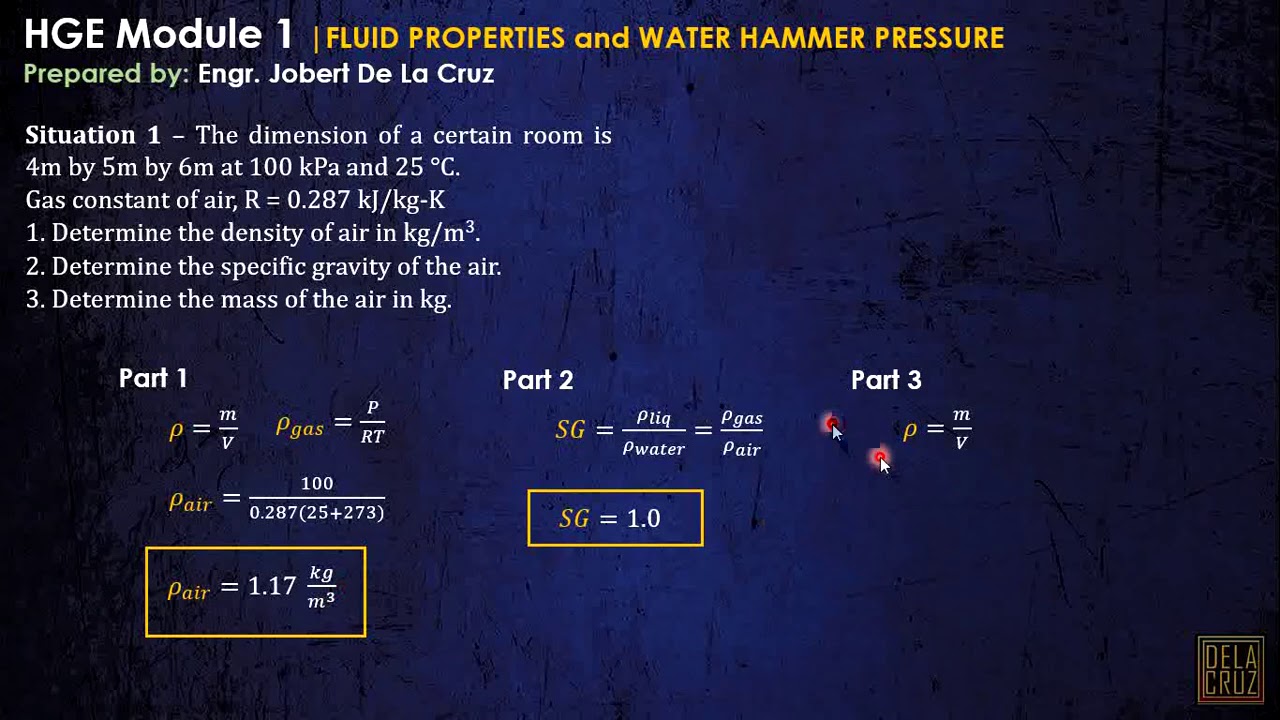
CE REVIEW - WEEK 1 | FLUID PROPERTIES

4.3b Pressure as Force per unit Area | AS Pressure | Cambridge A Level Physics
EXPLICANDO LA RELACION DE TRANSFORMACIÓN

Listrik Dinamis-Rangkaian Listrik (Hukum Ohm) (Part 3)

4.3c Fluid Pressure Equation and Graphs | AS Pressure | Cambridge A Level Physics
5.0 / 5 (0 votes)
