Regents Chemistry Nuclear Chemistry Part 3 Radioactive Decay
Summary
TLDRIn this engaging tutorial on nuclear chemistry, Dr. English explains the concept of radioactive decay, focusing on alpha decay, beta decay, and positron emission. The video outlines how unstable nuclei spontaneously decay into more stable forms, releasing radiation in the process. Dr. English demonstrates each type of decay with clear examples, emphasizing the conservation of mass and atomic numbers. Viewers are encouraged to participate with practice problems, enhancing their understanding of these fundamental nuclear processes. This informative session offers a solid foundation for anyone interested in the intricacies of radioactive decay.
Takeaways
- 😀 Radioactive decay occurs when an unstable nucleus spontaneously decays to form more stable products.
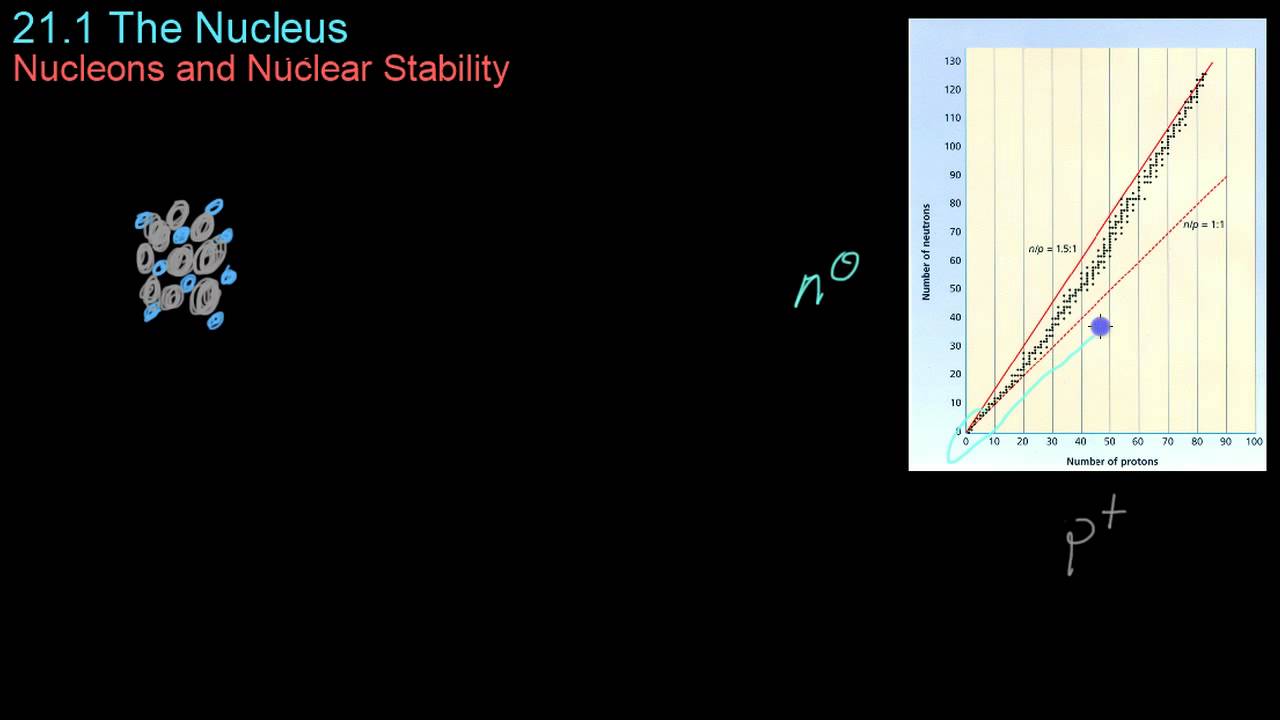
- 😀 The stability of a nucleus is often related to the ratio of protons to neutrons, ideally close to 1:1.
- 😀 Alpha decay involves the emission of an alpha particle, resulting in a decrease of the atomic number by 2 and the mass number by 4.
- 😀 The example of radium-226 undergoing alpha decay demonstrates the conservation of mass and atomic numbers in nuclear reactions.
- 😀 In beta decay, a neutron transforms into a proton, emitting a beta particle, which increases the atomic number by 1 without changing the mass number.
- 😀 The iodine-131 example illustrates how to balance nuclear equations during beta decay, maintaining the total mass number.
- 😀 Positron emission is the conversion of a proton into a neutron, resulting in the emission of a positron, which decreases the atomic number by 1.
- 😀 The potassium-37 to argon-37 conversion is used to explain positron emission and the conservation of mass number.
- 😀 Students are encouraged to practice solving for unknown elements in nuclear decay equations to reinforce their understanding.
- 😀 The tutorial covers key concepts of radioactive decay, including alpha decay, beta decay, and positron emission, providing a foundational understanding of nuclear chemistry.
Q & A
What is radioactive decay?
-Radioactive decay is the process by which an unstable nucleus spontaneously decays and forms more stable products, releasing radiation in the form of alpha particles, beta particles, positrons, and gamma radiation.
How does alpha decay affect the atomic number and mass number?
-In alpha decay, the atomic number decreases by 2 (as 2 protons are emitted with the alpha particle), and the mass number decreases by 4 (due to the loss of 2 protons and 2 neutrons).
What is an example of alpha decay provided in the transcript?
-An example given is the decay of radium-226 (Ra-226) to radon-222 (Rn-222), where the alpha particle is released, resulting in a more stable daughter nucleus.
What happens during beta decay?
-In beta decay, a neutron transforms into a proton and emits a beta particle (an electron), which results in an increase of the atomic number by 1, while the mass number remains unchanged.
Can you provide an example of beta decay from the script?
-The script mentions iodine-131 (I-131) undergoing beta decay, where it remains with the same mass number of 131, but its atomic number changes from 53 to 54, resulting in xenon-131 (Xe-131).
What is positron emission, and how does it affect the atomic number?
-Positron emission occurs when a proton is converted into a neutron, emitting a positron. This decreases the atomic number by 1 while keeping the mass number unchanged.
What example is used to illustrate positron emission?
-Potassium-37 (K-37) decays to argon-37 (Ar-37) through positron emission, where the mass number remains 37, but the atomic number decreases from 19 to 18.
How can we confirm the conservation of mass and atomic number during nuclear decay?
-We confirm conservation by ensuring that the sum of the mass numbers and atomic numbers on both sides of the nuclear equation is equal, indicating that mass and charge are conserved.
What is the relationship between proton-to-neutron ratio and nuclear stability?
-A proton-to-neutron ratio close to 1:1 generally indicates a more stable nucleus. When this ratio is significantly different, the nucleus is more likely to be unstable and undergo radioactive decay.
What should you do if you need more help understanding radioactive decay?
-If you need more help, the transcript encourages contacting the instructor for assistance.
Outlines

Cette section est réservée aux utilisateurs payants. Améliorez votre compte pour accéder à cette section.
Améliorer maintenantMindmap

Cette section est réservée aux utilisateurs payants. Améliorez votre compte pour accéder à cette section.
Améliorer maintenantKeywords

Cette section est réservée aux utilisateurs payants. Améliorez votre compte pour accéder à cette section.
Améliorer maintenantHighlights

Cette section est réservée aux utilisateurs payants. Améliorez votre compte pour accéder à cette section.
Améliorer maintenantTranscripts

Cette section est réservée aux utilisateurs payants. Améliorez votre compte pour accéder à cette section.
Améliorer maintenant5.0 / 5 (0 votes)