21.1 The Nucleus
Summary
TLDRThis video explores nuclear chemistry, covering key concepts such as nucleons, nuclear stability, and binding energy. It explains how mass defect is related to nuclear binding energy and the role of protons and neutrons in determining stability. The video discusses the band of stability and how the ratio of protons to neutrons shifts in larger elements. It also introduces the nuclear shell model, explaining the significance of 'magic numbers' for stable nuclei. Finally, it touches on nuclear reactions, including fusion and transmutation, and highlights the importance of conserving mass and charge in these processes.
Takeaways
- 😀 Nucleons refer to protons and neutrons, the particles that make up an atom's nucleus.
- 😀 A nucleide is a term used for an atom in nuclear chemistry, often excluding the electron cloud.
- 😀 The shorthand notation for writing a nucleide involves its mass number (total nucleons) and atomic number (protons).
- 😀 Mass defect is the difference between the expected mass of an atom and its actual mass, important for understanding nuclear binding energy.
- 😀 The nuclear binding energy is the energy required to create a nucleus or to break it apart, and it is derived from the mass defect using Einstein’s equation E=mc².
- 😀 The higher the nuclear binding energy, the more stable the nucleus is, as more energy is needed to break it apart.
- 😀 The band of stability shows that smaller nuclei have a 1:1 ratio of protons to neutrons, while larger nuclei require more neutrons to remain stable.
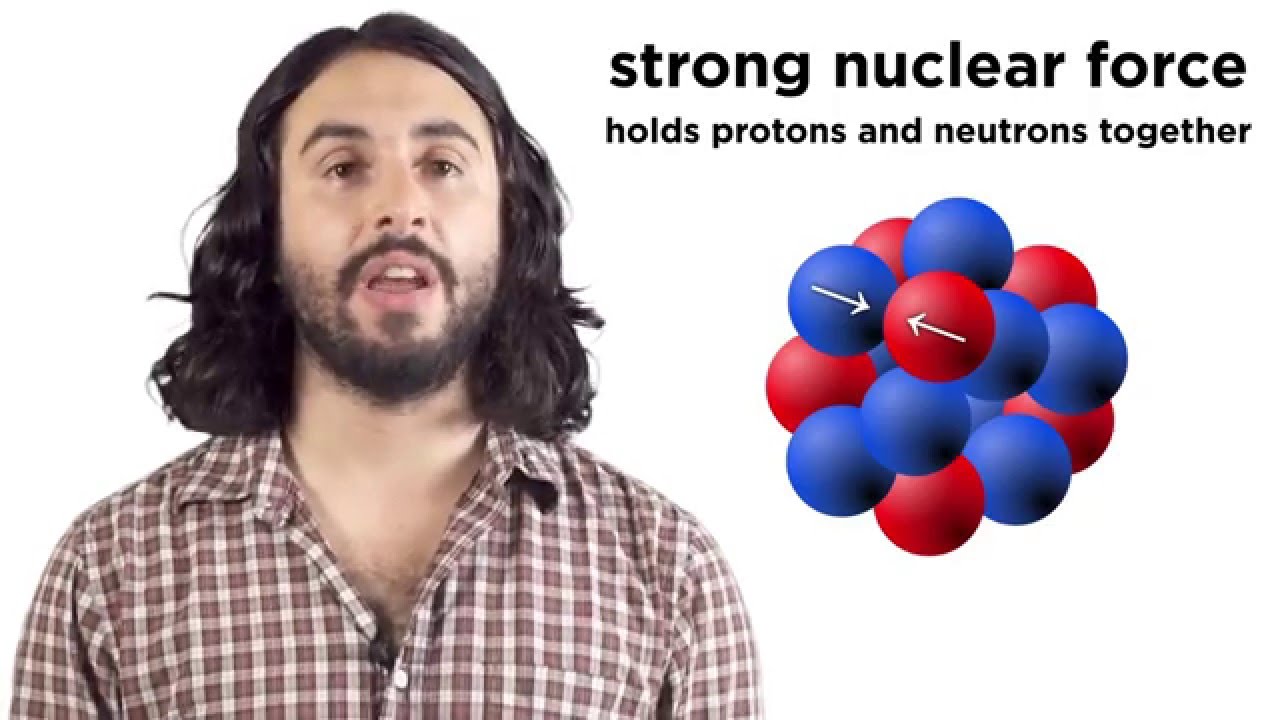
- 😀 The strong nuclear force is responsible for holding nucleons together in the nucleus, but its effect diminishes with distance, necessitating more neutrons in larger nuclei.
- 😀 The nuclear shell model suggests that nuclei with certain 'magic numbers' of nucleons (2, 8, 20, 28, 50, 82, 126) are especially stable.
- 😀 Nuclear reactions involve changes to the nucleus and can lead to transmutation, where one element is converted into another, as seen in fusion reactions.
- 😀 Transmutation in nuclear fusion is an example where the mass and charge are conserved, resulting in a new element, such as the fusion of beryllium and helium to form carbon.
Q & A
What is a nucleon?
-A nucleon is a collective term for protons and neutrons, which are the components of an atomic nucleus.
Why is a 'nuide' used in nuclear chemistry?
-A 'nuide' is another term for an atom in nuclear chemistry, used to focus specifically on the nucleus and exclude the electron cloud of the atom.
How are different nuclides typically written in shorthand notation?
-Nuclides are written with their mass number (total number of nucleons) as a superscript and their atomic number (number of protons) as a subscript. For example, radium-228 is written as 228/88 Ra.
What is the mass defect?
-The mass defect is the difference between the expected mass (the sum of the individual nucleons' masses) and the actual mass of the nucleus. This discrepancy is due to the energy required to hold the nucleus together.
How is the nuclear binding energy calculated?
-Nuclear binding energy is calculated using Einstein's equation, E = mc^2, where m is the mass defect, and c is the speed of light. This energy represents the energy required to disassemble a nucleus.
Why is a higher nuclear binding energy indicative of greater stability?
-A higher nuclear binding energy means more energy is needed to break the nucleus apart, indicating that the nucleus is more stable and less likely to undergo spontaneous decay.
What does the band of stability represent on the plot of protons versus neutrons?
-The band of stability shows the range of proton-to-neutron ratios that correspond to stable isotopes. For small nuclei, the ratio is about 1:1, but for larger nuclei, it increases to approximately 1.5:1.
What is the strong nuclear force, and why is it important for nuclear stability?
-The strong nuclear force is the force that binds protons and neutrons together within the nucleus. It operates at very short ranges and helps counteract the electrostatic repulsion between positively charged protons, maintaining nuclear stability.
What is the nuclear shell model?
-The nuclear shell model is a theory that explains the stability of nuclei based on the arrangement of nucleons (protons and neutrons) in shells. It is similar to the electron shell model in atomic physics, with 'magic numbers' (2, 8, 20, 28, 50, 82, 126) indicating particularly stable configurations.
What is nuclear transmutation?
-Nuclear transmutation is the process in which one element is converted into another, typically through nuclear reactions like fusion. This can occur spontaneously or artificially in laboratories.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowBrowse More Related Video
5.0 / 5 (0 votes)