Konsentrasi Larutan bag 1
Summary
TLDRIn this educational video, Broery Pancawati explains the fundamentals of solution concentrations, focusing on concepts such as mass percent, volume percent, and various methods of calculating them. The lesson uses relatable examples, like preparing a saline solution and measuring alcohol content in mixtures, to illustrate how to derive concentrations. It highlights the importance of balance in ingredient ratios and provides step-by-step guidance on solving concentration problems, making it accessible for learners. By the end, viewers gain a clear understanding of solution chemistry and its practical applications.
Takeaways
- ☕ Making a cup of coffee involves mixing coffee powder, sugar, and hot water in the right proportions.
- ⚖️ The balance between solute (like sugar) and solvent (like water) affects the taste and concentration of the solution.
- 📊 Concentration of a solution refers to the amount of solute in a given amount of solvent.
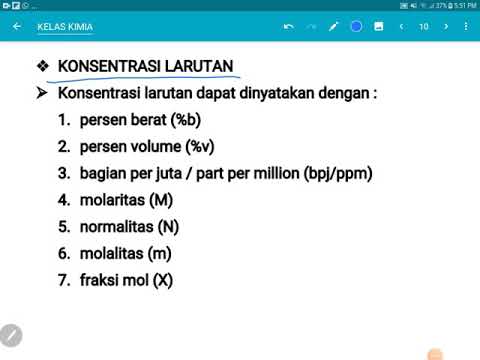
- 📈 There are six types of concentration known: percent concentration, molarity, normality, molality, and others.
- 📏 Percent concentration can be calculated in three ways: percent by volume, percent by mass, and percent by mass per volume.
- 🔍 Percent by mass is calculated as the mass of solute divided by the total mass of the solution, multiplied by 100.
- 🌊 Example calculations illustrate how to determine percent mass concentration using saltwater solutions.
- 💧 Percent by volume indicates the volume of solute in a total volume of 100 mL of solution.
- 🧮 Formulas for calculating concentration provide a clear method for solving practical chemistry problems.
- 📚 Understanding these concepts is essential for practical applications in chemistry and cooking.
Q & A
What is the main topic discussed in the transcript?
-The main topic is the concentration of solutions, focusing on various methods of expressing concentration, such as mass percentage and volume percentage.
What are the key components needed to make a cup of coffee according to the transcript?
-To make a cup of coffee, you need coffee powder and sugar, combined with hot water.
How is the concentration of a solution defined?
-Concentration of a solution is defined as the amount of solute present in a given amount of solvent or solution.
What are the six types of concentration mentioned in the lesson?
-The six types of concentration are weight percentage, molarity, normality, molality, and percent weight/volume.
How is percent mass concentration calculated?
-Percent mass concentration is calculated as (mass of solute / total mass of solution) x 100%.
In the first example, how much salt was dissolved in water?
-In the first example, 30 grams of table salt (NaCl) was dissolved in 120 grams of water.
What formula is used to find the total mass of a solution?
-The total mass of the solution is found by adding the mass of the solute to the mass of the solvent.
What is the significance of volume percentage in solutions?
-Volume percentage indicates the volume of solute in 100 milliliters of solution, providing a way to express concentration in terms of volume.
How is volume percentage calculated?
-Volume percentage is calculated using the formula: (volume of solute / total volume of solution) x 100%.
What was the final concentration of acetic acid in the second example?
-In the second example, the concentration of acetic acid (CH3COOH) in the solution was calculated to be 2.5%.
Outlines

Esta sección está disponible solo para usuarios con suscripción. Por favor, mejora tu plan para acceder a esta parte.
Mejorar ahoraMindmap

Esta sección está disponible solo para usuarios con suscripción. Por favor, mejora tu plan para acceder a esta parte.
Mejorar ahoraKeywords

Esta sección está disponible solo para usuarios con suscripción. Por favor, mejora tu plan para acceder a esta parte.
Mejorar ahoraHighlights

Esta sección está disponible solo para usuarios con suscripción. Por favor, mejora tu plan para acceder a esta parte.
Mejorar ahoraTranscripts

Esta sección está disponible solo para usuarios con suscripción. Por favor, mejora tu plan para acceder a esta parte.
Mejorar ahoraVer Más Videos Relacionados

Solutions, Percent by Mass and Volume
Kelas Kimia : Konsentrasi Larutan (% berat, % volume, ppm / bpj)

Expressing the Concentration of Solutions | Chemistry

Concentração das Soluções em Porcentagem

Lab 6 - Weight-Average Mass Of Paperclipium (A/E Chem Virtual Lab)

Very Important formulas for calculation of Moles ||For class 9th, First year|| for ETEA and NMCAT
5.0 / 5 (0 votes)
