Expressing the Concentration of Solutions | Chemistry
Summary
TLDRTeacher Jane from Teach Talk explains how to express the concentrations of solutions in various forms, including percent by mass, percent by volume, degree proof, and parts per million. She provides clear examples and formulas for each method, making complex scientific concepts accessible and engaging for students from grades 7 to 12.
Takeaways
- 📚 The video is an educational resource from 'Teach Talk', focusing on making learning enjoyable and straightforward.
- 🔔 It encourages viewers to subscribe and enable notifications for updates on future videos.
- 🌟 The video introduces various concepts for students from grades 7 to 12, covering a range of scientific topics including general science, biology, chemistry, physics, and earth science.
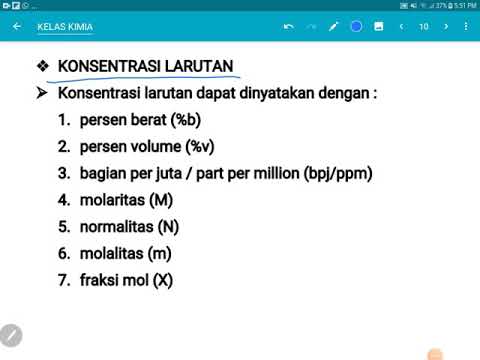
- 🧪 The main topic of the video is expressing the concentrations of solutions, which is defined as the ratio of the amount of solute to the amount of solution.
- 📏 The video explains different methods to express solution concentrations, such as percent by mass, percent by volume, parts per million (ppm), and parts per billion (ppb).
- 📉 For percent by mass, the formula is given as (mass solute / mass solution) x 100, and an example is provided to calculate the percentage of a sugar solution.
- 📊 The video also covers percent by volume, using a similar formula but with volumes instead of masses, and includes a sample problem involving alcohol in a solution.
- 🍷 Degree proof, a measure specific to alcohol concentration, is discussed, and it's noted that it's twice the percent by volume.
- 🔬 ppm and ppb are introduced for very small solute concentrations, with formulas provided for their calculation based on mass and volume.
- 📝 A short quiz is included at the end of the video to test the viewer's understanding of the concepts taught regarding solution concentration.
- 👋 The video concludes with a call to action for viewers to subscribe, like, and share the video, and it ends with a friendly farewell.
Q & A
What is the main topic discussed in the Teach Talk video?
-The main topic discussed in the Teach Talk video is expressing the concentrations of solutions, covering various methods such as percent by mass, percent by volume, degree proof, and parts per million or billion.
What does the term 'concentration' mean in the context of solutions?
-In the context of solutions, 'concentration' refers to the ratio of the amount of solute to the amount of the solution, which can be expressed as solute over solution.
How is percent by mass calculated?
-Percent by mass is calculated using the formula: (mass of solute / mass of solution) * 100.
Can you provide an example of how to calculate percent by mass from the script?
-An example from the script is a salt solution prepared by dissolving 6 grams of table salt in 50 grams of solution, resulting in a percent by mass of 12%.
What is the difference between percent by mass and percent by volume?
-Percent by mass is based on the mass of the solute and solution, while percent by volume is based on their volumes.
How do you calculate the percent by volume of a solution?
-Percent by volume is calculated using the formula: (volume of solute / volume of solution) * 100.
What is the degree proof and how is it related to percent by volume?
-Degree proof is a measure used to express the concentration of alcohol, typically calculated by multiplying the percent by volume by two.
When might parts per million (ppm) or parts per billion (ppb) be used to express concentration?
-Parts per million (ppm) or parts per billion (ppb) are used when the amount of solute in the solution is very small, requiring a more sensitive measure of concentration.
How is the concentration expressed in parts per million (ppm) calculated?
-Concentration in parts per million is calculated using the formula: (mass solute in milligrams / volume of solution in liters).
What is the importance of understanding different ways to express solution concentrations?
-Understanding different ways to express solution concentrations is important for accurate scientific communication, experimentation, and for applications in various fields such as chemistry, biology, and medicine.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowBrowse More Related Video
5.0 / 5 (0 votes)