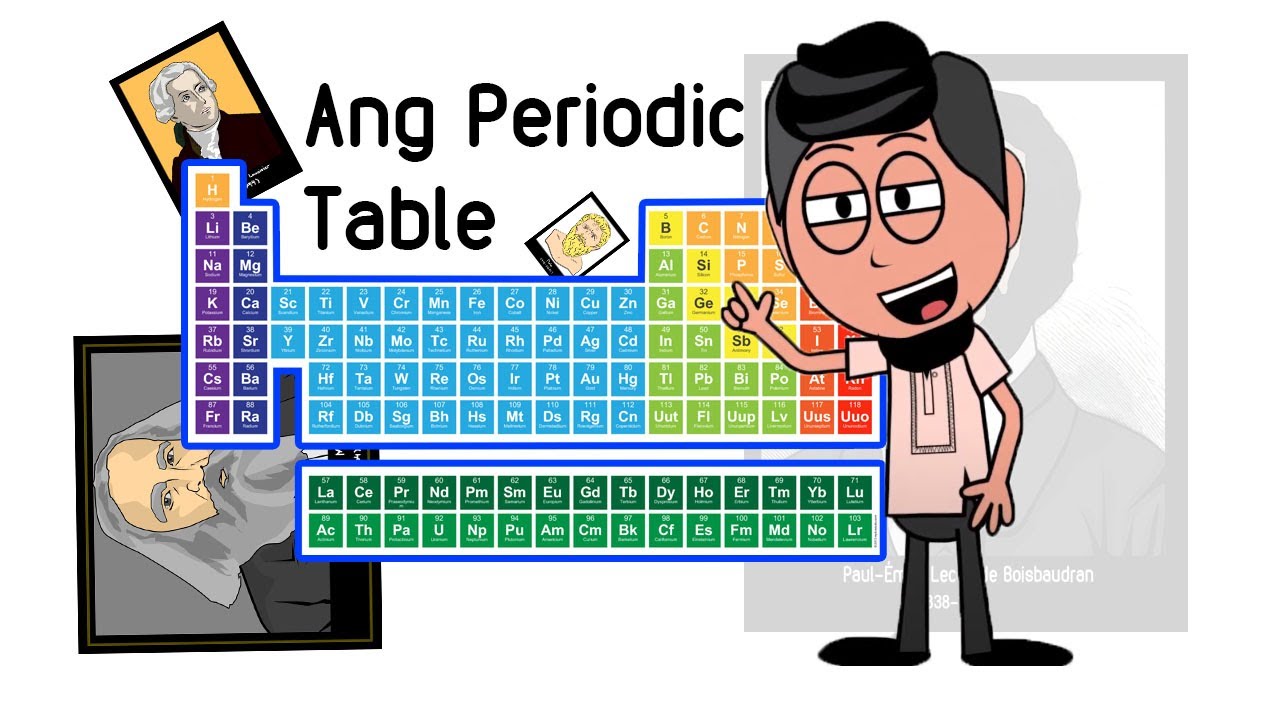
Sejarah Perkembangan Sistem Periodik Unsur
Summary
TLDRThis video discusses the periodic table of elements, tracing its historical development from ancient Greek theories to modern science. It highlights significant contributions from key figures like Robert Boyle, John Newlands, Dmitri Mendeleev, and Henry Moseley, illustrating how their theories advanced the understanding of elements and their organization. The conversation shifts from a lighthearted dialogue about personal relationships to a deeper inquiry into the nature of matter, emphasizing the periodic table's role in categorizing elements based on their properties and atomic numbers, ultimately showcasing its importance in chemistry.
Takeaways
- 😀 The periodic table is a significant achievement in chemistry, containing vital information about elements.
- 😀 Ancient Greek philosophers believed matter was made up of four elements: earth, water, air, and fire.
- 😀 Robert Boyle redefined elements in the 17th century as substances that cannot be broken down further.
- 😀 The 19th century saw many elements discovered through methods like electrolysis and spectroscopy.
- 😀 Questions arose about the limitations and patterns in the properties of elements as new discoveries were made.
- 😀 The triad theory proposed by Johann Döbereiner identified groups of three elements with similar properties but had limitations.
- 😀 John Newlands introduced the 'Law of Octaves' in 1864, suggesting periodic similarities every eighth element.
- 😀 Dmitri Mendeleev arranged elements based on their atomic mass and grouped those with similar properties, laying groundwork for the modern periodic table.
- 😀 Mendeleev's system had flaws, such as misplacements, but was innovative in leaving gaps for undiscovered elements.
- 😀 Henry Moseley’s work in 1914 established the periodic table should be organized by atomic number rather than atomic mass, refining Mendeleev's original model.
Q & A
What is the main topic discussed in the transcript?
-The transcript discusses the concept of elements and the periodic table, including its history and the properties of different elements.
What are the four classical elements proposed by ancient Greek philosophers?
-The four classical elements are earth, water, air, and fire.
Who was Robert Boyle and what was his contribution to the understanding of elements?
-Robert Boyle was a 17th-century chemist who defined an element as a substance that cannot be broken down into simpler substances, establishing a more modern understanding of chemical elements.
What significant advancement did Dmitri Mendeleev make in the 19th century?
-Dmitri Mendeleev created the first periodic table in 1869, organizing elements by increasing atomic mass and grouping them by similar properties.
What is the significance of atomic number in the modern periodic table?
-In the modern periodic table, elements are arranged by atomic number rather than atomic mass, which provides a more accurate representation of their properties.
What was the 'Law of Octaves' proposed by John Newlands?
-John Newlands proposed the 'Law of Octaves' in 1864, stating that elements arranged by increasing atomic mass show similar properties at intervals of eight.
What limitations did Mendeleev's periodic table have?
-Mendeleev's periodic table sometimes placed elements out of order by mass and left gaps for undiscovered elements, which indicated that there were elements yet to be found.
Who is Henry Moseley and what did he contribute to the periodic table?
-Henry Moseley was a scientist who, in the early 20th century, rearranged the periodic table by atomic number instead of atomic mass, correcting inaccuracies in Mendeleev's system.
How do elements relate to the concept of atoms?
-Elements are pure substances made up of only one type of atom, and all matter in the universe is composed of these atoms.
What are the two main types of properties used to categorize elements in the periodic table?
-Elements in the periodic table are categorized based on their physical properties (like state and color) and chemical properties (such as reactivity and bonding behavior).
Outlines

Esta sección está disponible solo para usuarios con suscripción. Por favor, mejora tu plan para acceder a esta parte.
Mejorar ahoraMindmap

Esta sección está disponible solo para usuarios con suscripción. Por favor, mejora tu plan para acceder a esta parte.
Mejorar ahoraKeywords

Esta sección está disponible solo para usuarios con suscripción. Por favor, mejora tu plan para acceder a esta parte.
Mejorar ahoraHighlights

Esta sección está disponible solo para usuarios con suscripción. Por favor, mejora tu plan para acceder a esta parte.
Mejorar ahoraTranscripts

Esta sección está disponible solo para usuarios con suscripción. Por favor, mejora tu plan para acceder a esta parte.
Mejorar ahoraVer Más Videos Relacionados
5.0 / 5 (0 votes)