Review of: History of an atom & Ions
Summary
TLDRThis video covers the history and development of atomic theory, including key milestones from ancient philosophies to modern scientific discoveries. The instructor reviews the structure of atoms, the formation of ions, and explains the concept of isotopes. The lesson also discusses upcoming MAP testing and final exams, along with assigned asynchronous work. The video emphasizes the evolving nature of scientific understanding, particularly in relation to atomic models, from early theories to the widely accepted modern model.
Takeaways
- 📅 This video covers the January calendar, focusing on topics like the history and structure of atoms, ions, and important dates for map testing and final exams.
- 🔬 The history of atomic theory is discussed, beginning with ancient Indian philosophers who believed matter couldn't be continuously divided.
- 🧠 Democritus contributed to the atomic theory, suggesting that all matter is made of small particles with space between them around 460 BCE.
- 🌐 The concept of atoms continued to evolve, with Buddhist philosophers in 600 AD proposing that atoms are small, eternal points made of energy.
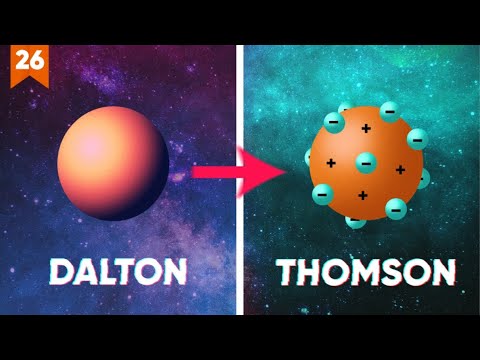
- 🧪 John Dalton's modern atomic theory from 1808 AD introduced five key ideas, including the indivisibility of atoms and their combinations in whole-number ratios.
- ⚛️ JJ Thompson's discovery in 1897 AD showed that atoms could be broken down into smaller parts, leading to the plum pudding model.
- 🔍 Ernest Rutherford's 1911 experiment revealed that atoms are mostly empty space with a positively charged nucleus at the center.
- 🔄 Niels Bohr refined Rutherford's model in 1913 by describing electrons orbiting the nucleus in fixed sizes and energies.
- ☁️ Erwin Schrodinger's model from 1926 described electrons as moving in waves, creating a probability cloud around the nucleus.
- 🧲 The video also reviews ions, explaining that atoms gain or lose electrons to form ions, which have either positive or negative charges.
Q & A
What is the significance of the dates BCE and CE in the context of scientific discoveries?
-BCE (Before Common Era) and CE (Common Era) are used to denote the dating system in historical contexts. BCE refers to years before the year 1 AD, with the numbering increasing as you go further back in time (e.g., 2000 BCE is approximately 4000 years ago). CE refers to years after the year 1 AD, with the numbering increasing as you go forward in time.
Who is credited with one of the earliest beginnings of the atomic theory?
-Democritus is often given credit for one of the first beginnings of the atomic theory, with his ideas documented around 460 BCE. He proposed that all matter was made up of atoms with lots of space in between them.
What is the modern atomic theory according to John Dalton?
-John Dalton's modern atomic theory, proposed in 1803, states that all matter is made up of atoms, atoms are indivisible, atoms of a given element are identical, compounds are combinations of different types of atoms, and atoms combine in simple whole number ratios.
What discovery did J.J. Thomson make in 1897 that added to the understanding of atoms?
-J.J. Thomson discovered electrons in 1897 through an experiment that created a beam of electrons. He used a magnet to change the beam's direction, leading to the plum pudding model of the atom, which included the idea of an atom being made up of positive and negative portions.
What did Ernest Rutherford's gold foil experiment reveal about the structure of the atom?
-Ernest Rutherford's gold foil experiment revealed that atoms are mostly empty space with a small, dense, positively charged center, which he identified as the nucleus. Some particles deflected or bounced back, indicating the presence of a concentrated positive charge.
How did Niels Bohr revise Rutherford's model of the atom?
-Niels Bohr revised Rutherford's model by suggesting that electrons move around the nucleus in orbits of fixed size and energy. Electrons at specific energy levels could not move between these orbits without gaining or losing energy.
What is the significance of Erwin Schrödinger's discovery regarding electron movement?
-Erwin Schrödinger discovered that electrons do not move in set paths but rather in waves, indicating that their exact locations cannot be known. His model introduced the concept of a probability cloud, showing that electrons are in constant motion with their energy levels changing depending on their location.
What is an ion and how is it created?
-An ion is an atom that has gained or lost electrons, resulting in a net positive or negative charge. This change in the number of electrons alters the atom's charge, making it an ion.
What is the difference between isotopes and how are they formed?
-Isotopes are atoms of the same element that have the same number of protons but different numbers of neutrons. They are formed due to variations in the number of neutrons within the atomic nucleus.
What is the net charge of an atom and how does it relate to the number of protons and electrons?
-The net charge of an atom is determined by the balance between the number of protons (positive charges) and electrons (negative charges). If the number of protons equals the number of electrons, the atom is neutral. If there are more protons than electrons, the atom has a positive charge, and if there are more electrons than protons, it has a negative charge.
What is the educational purpose of the warm-up posted in Schoology for the week of January 11th?
-The warm-up posted in Schoology for the week of January 11th is designed to help students review and understand the history of the atom and the development of atomic theories over time, as well as to practice ordering scientific events from oldest to newest.
Outlines

Esta sección está disponible solo para usuarios con suscripción. Por favor, mejora tu plan para acceder a esta parte.
Mejorar ahoraMindmap

Esta sección está disponible solo para usuarios con suscripción. Por favor, mejora tu plan para acceder a esta parte.
Mejorar ahoraKeywords

Esta sección está disponible solo para usuarios con suscripción. Por favor, mejora tu plan para acceder a esta parte.
Mejorar ahoraHighlights

Esta sección está disponible solo para usuarios con suscripción. Por favor, mejora tu plan para acceder a esta parte.
Mejorar ahoraTranscripts

Esta sección está disponible solo para usuarios con suscripción. Por favor, mejora tu plan para acceder a esta parte.
Mejorar ahoraVer Más Videos Relacionados

The Atomic Theory: A Timeline Through History

A Estrutura do Átomo - 1/6 - Os primeiros modelos

Química - Natureza elétrica da matéria e núcleo atômico (prof. Luiz Landim)
O Modelo Atômico de Dalton x Thomson

Kimia X - Struktur Atom #3 | Perkembangan Teori dan Model Atom

A History of Our Knowledge of the Solar System
5.0 / 5 (0 votes)
