Química - Natureza elétrica da matéria e núcleo atômico (prof. Luiz Landim)
Summary
TLDRThis video script covers the evolution of atomic theory, starting with ancient Greek ideas about matter and moving through key scientific models. It discusses Dalton's early atomic model and its limitations, followed by Thomson's discovery of electrons through experiments with cathode rays. The script then delves into Rutherford’s groundbreaking gold foil experiment, which revealed the nucleus's existence and the atom's structure. The discussion concludes with a look at the atomic model of Rutherford, which remains foundational to modern atomic theory, along with a mention of later discoveries like neutrons by Chadwick.
Takeaways
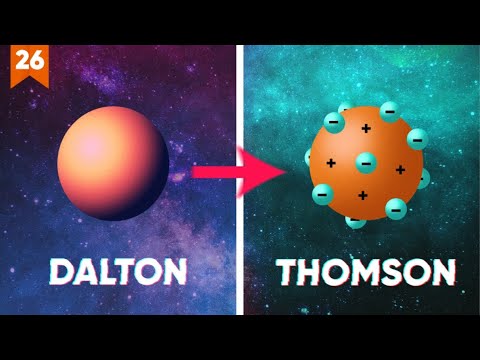
- 😀 Dalton's atomic model suggested that atoms were indivisible and that elements differed in size and mass of their atomic spheres. However, Dalton failed to explain the electrical nature of matter.
- 😀 Thomson's atomic model introduced the concept of negatively charged electrons embedded in a positively charged sphere, explaining the electrical nature of matter.
- 😀 The analogy of the 'ball of ice cream with raisins' was used to illustrate Thomson's model, where the 'raisins' (electrons) were embedded in the 'ice cream' (positively charged matter).
- 😀 Dalton's model failed because it couldn't explain the presence of electrical charges in matter, an issue that was later addressed by Thomson's model.
- 😀 Thomson's model was eventually replaced by Rutherford's model after a key experiment using alpha particles, which disproved Thomson's concept of a uniform positive charge.
- 😀 Rutherford's experiment showed that atoms have a dense, positively charged nucleus and a vast, empty space around it where electrons orbit, leading to the 'planetary model'.
- 😀 In Rutherford's experiment, most alpha particles passed straight through the gold foil, while some were deflected, suggesting that atoms consist mostly of empty space, with a small dense nucleus.
- 😀 Rutherford's findings led to the conclusion that the atom has a small, dense nucleus that contains positive charge, and electrons orbit around this nucleus.
- 😀 The concept of protons was confirmed by Rutherford's model, though the discovery of neutrons was later credited to James Chadwick.
- 😀 The model of the atom evolved over time, with Dalton's indivisible atom theory being debunked, Thomson introducing electrons, and Rutherford demonstrating the nucleus, laying the groundwork for future atomic theory.
Q & A
What is the primary focus of the script?
-The script discusses the evolution of atomic models, starting from the ancient Greek idea of atoms to the modern understanding of atomic structure, including the failures of early atomic models like Dalton's and Thomson's, and the experimental evidence that led to Rutherford's planetary model.
What was Dalton's model of the atom, and what were its limitations?
-Dalton's model proposed that atoms were indivisible, solid spheres, and the difference between elements was due to the size and mass of these spheres. However, it failed to explain the electrical nature of matter, a key limitation that was later addressed by other scientists.
Why did Dalton's atomic model fail?
-Dalton's model failed because it could not explain the electrical nature of matter. Although electricity had been discovered, Dalton's theory did not account for the existence of subatomic particles like electrons, which was crucial for understanding the electrical properties of matter.
How did Thomson's model of the atom differ from Dalton's?
-Thomson's model, known as the 'plum pudding' model, proposed that the atom was a positive sphere with negatively charged electrons embedded in it. This was a departure from Dalton's indivisible spheres, offering an explanation for the electrical nature of atoms.
What experiment did Thomson conduct to support his model?
-Thomson used cathode ray tubes to demonstrate the presence of negatively charged particles, later identified as electrons, embedded within the positively charged atomic sphere. This experiment helped confirm the idea that atoms contained subatomic particles.
What was the key flaw in Thomson's atomic model?
-The key flaw in Thomson's model was that it could not explain the structure of the atom in a way that accounted for the results of subsequent experiments, particularly Rutherford's gold foil experiment, which showed that the atom had a dense, positively charged nucleus.
What was the gold foil experiment conducted by Rutherford, and what did it reveal?
-Rutherford's gold foil experiment involved shooting alpha particles at a thin sheet of gold. Most particles passed through, but some were deflected, revealing that the atom had a small, dense, positively charged nucleus, and that most of the atom was empty space. This disproved Thomson's model.
How did Rutherford explain the results of his gold foil experiment?
-Rutherford explained the results by proposing that atoms consist of a tiny, dense nucleus containing positive charge (later understood as protons), surrounded by a much larger region of empty space where the electrons are located. This led to the planetary model of the atom.
What are the key components of Rutherford's atomic model?
-Rutherford's model posited that the atom has a small, dense nucleus that contains positively charged protons, and electrons orbit this nucleus in a manner similar to planets orbiting the sun. The majority of the atom is empty space.
How did the discovery of neutrons impact the atomic model after Rutherford's work?
-The discovery of neutrons by James Chadwick later added to Rutherford's model. Neutrons, which are neutral particles found in the nucleus alongside protons, helped refine the understanding of atomic structure, explaining the mass of atoms more accurately.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowBrowse More Related Video

MODELOS ATÔMICOS: Dalton, Thomson, Rutherford e Rutherford-Bohr

IPA Kelas 9 Semester 2 : Partikel Penyusun Materi (Part 1 : atom dan molekul)

PERKEMBANGAN TEORI ATOM | Dalton, Thomson, Rutherford, Bohr, Heisenberg, Erwin Schrodinger,
O Modelo Atômico de Dalton x Thomson

Atomic Structure | Grade 8 Science DepEd MELC Quarter 3 Module 3

The History of Atomic Chemistry: Crash Course Chemistry #37
5.0 / 5 (0 votes)