The Photoelectric Effect - A Level Physics
Summary
TLDRThe video delves into the photoelectric effect, a pivotal discovery in physics. It traces the phenomenon from Heinrich Hertz's 1887 observation of ultraviolet light causing sparks on electrodes, to Einstein's groundbreaking 1905 explanation. Einstein's work showed that light behaves as both a wave and a particle, with photons causing electrons to be emitted from a material when they have sufficient energy. The video uses a gold leaf electroscope to demonstrate the effect and highlights the concept of the 'work function,' the energy required for electrons to escape an atom. This discovery laid the foundation for quantum mechanics.
Takeaways
- 😀 The photoelectric effect was first observed in 1887 by Heinrich Hertz, who found that ultraviolet light caused increased sparking between two electrodes.
- 😀 In 1905, Albert Einstein provided a groundbreaking explanation for the photoelectric effect, for which he won the Nobel Prize.
- 😀 Einstein's explanation of the photoelectric effect showed that electrons can be emitted by photons (light particles) when they collide with a material.
- 😀 The gold leaf electroscope is a device that demonstrates the photoelectric effect by showing how light can discharge an electrically charged system.
- 😀 When red light was shone on a charged zinc plate in an electroscope, no electrons were emitted, but ultraviolet light caused electron emission, illustrating the photoelectric effect.
- 😀 The wave model of light could not explain why only specific wavelengths (like ultraviolet) caused electron emission, leading to the development of a particle model for light.
- 😀 Einstein proposed that light is quantized into particles called photons, and only photons with enough energy (like ultraviolet light) could release electrons from atoms.
- 😀 For an electron to escape an atom, it needs to absorb energy from a photon. If the photon's energy is too low, the electron will not be emitted.
- 😀 The energy required for an electron to escape an atom is known as the 'work function,' a concept introduced by Einstein to describe the energy barrier an electron must overcome.
- 😀 Different metals have different work functions, meaning that the energy required to release an electron varies depending on the material.
- 😀 The energy of the incoming photon is transferred to the electron, and if any energy remains after the electron escapes, it is converted into the kinetic energy of the electron, resulting in a photoelectron.
Q & A
What discovery did Heinrich Hertz make in 1887 regarding ultraviolet light?
-In 1887, Heinrich Hertz discovered that shining ultraviolet light on two electrodes caused them to spark more often than when no light was present. This phenomenon was later explained by the photoelectric effect.
What was the significance of Einstein's work on the photoelectric effect?
-Einstein's work on the photoelectric effect, for which he won the Nobel Prize in Physics in 1921, explained how photons can cause electrons to be emitted from a metal surface. His work showed that light has particle-like properties, not just wave-like, which was a breakthrough in understanding the behavior of light.
How does a gold leaf electroscope demonstrate the photoelectric effect?
-A gold leaf electroscope can be used to demonstrate the photoelectric effect by showing how electrons are emitted from a metal surface when exposed to light. The electroscope's gold leaf moves in response to changes in charge, which occurs when ultraviolet light causes electrons to be ejected from the zinc plate, reducing the charge.
Why does shining red light on the zinc plate in the electroscope not cause a discharge?
-Shining red light on the zinc plate does not cause a discharge because the energy of red photons is too low to free the electrons from the metal. According to the photoelectric effect, only photons with sufficient energy (like ultraviolet light) can cause electron emission.
What happens when ultraviolet light is used in the electroscope demonstration?
-When ultraviolet light is used, even at low intensities, it causes electrons to be emitted from the metal surface of the electroscope. The emission of these electrons reduces the charge on the electroscope, causing the gold leaf to fall, which demonstrates the photoelectric effect.
Why couldn’t the wave model of light explain the photoelectric effect?
-The wave model of light couldn't explain the photoelectric effect because it suggested that the energy from light would accumulate over time, gradually releasing electrons. However, experiments showed that only light of a certain frequency (and therefore energy) could cause electron emission, not the total intensity or brightness of light.
What was Einstein’s explanation for the photoelectric effect?
-Einstein explained that light consists of particles called photons. For an electron to be emitted from a metal, it must absorb a photon with enough energy to overcome the electron's binding energy in the atom. If the photon’s energy is sufficient, the electron is emitted, and any excess energy is converted into kinetic energy of the ejected electron.
What is the 'work function' in the context of the photoelectric effect?
-The 'work function' refers to the minimum amount of energy required to release an electron from the surface of a material. Different materials have different work functions, which determines whether light of a given energy (such as red or ultraviolet light) can cause electrons to be emitted.
How does the energy of a photon relate to the emission of a photoelectron?
-The energy of the incoming photon must be sufficient to overcome the work function of the material for an electron to be emitted. If the photon’s energy is greater than the work function, the excess energy is transferred to the electron as kinetic energy, which is why the emitted electron is called a photoelectron.
Why do different metals have different responses to light in the photoelectric effect?
-Different metals have different work functions, meaning they require different amounts of energy to release electrons. Some metals may allow red light (low energy) to release electrons, while others may require more energetic light, like ultraviolet, to achieve the same effect.
Outlines

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنMindmap

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنKeywords

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنHighlights

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنTranscripts

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنتصفح المزيد من مقاطع الفيديو ذات الصلة

#Fisikapopuler Eps. 1: Sejarah Lahirnya Fisika Kuantum

Modern Physics: an overview of key themes as a concept map
What is Compton Scattering?
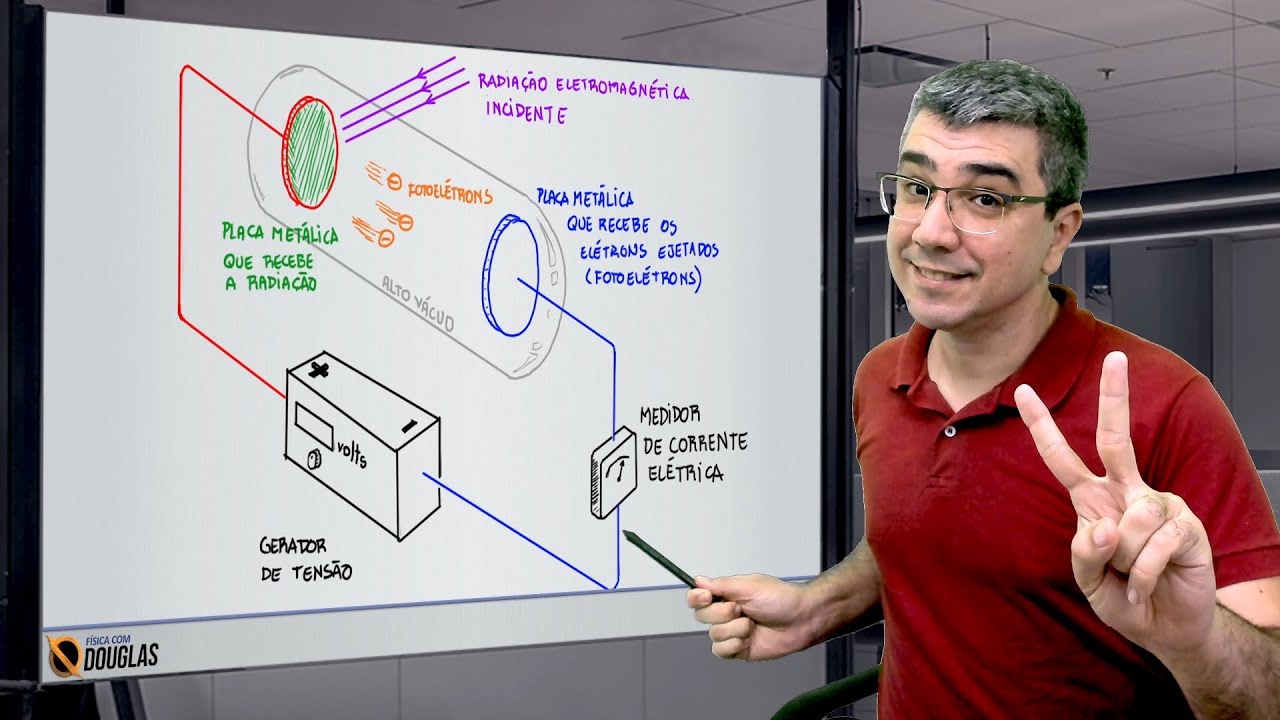
Física quântica - Efeito fotoelétrico Parte 2 de 2

Physics - Photoelectric effect

The Quantum Theory of the Atom | Chapter 7 - General, Organic, and Biological Chemistry
5.0 / 5 (0 votes)
