Substitusi Elektrofilik Aromatik: Sulfonasi
Summary
TLDRThis video provides an overview of electrophilic aromatic substitution, specifically focusing on the sulfonation reaction. The process involves reacting aromatic rings with sulfur trioxide (SO3) in the presence of concentrated sulfuric acid to form sulfonic acid derivatives. The mechanism includes the formation of the electrophile SO3H+, its attack on the aromatic ring to form a resonance-stabilized intermediate, and subsequent restoration of aromaticity. The video also covers practical applications of sulfonation, such as in the production of dyes and sulfa drugs like sulfanilamide, and offers an example exercise on benzene derivatives.
Takeaways
- 😀 Sulfonation is an electrophilic aromatic substitution reaction involving an aromatic ring and fuming sulfuric acid (H2SO4 + SO3).
- 😀 Fuming sulfuric acid is a mixture of concentrated sulfuric acid and sulfur trioxide (SO3), which acts as the electrophile in the reaction.
- 😀 The reaction forms a sulfonic acid group (-SO3H) attached to the aromatic ring.
- 😀 The first step of the sulfonation mechanism involves the formation of the electrophile SO3H+ from SO3 and H2SO4.
- 😀 In the second step, the electrophile (SO3H+) attacks the benzene ring, leading to the formation of an aryl cation (arynium ion).
- 😀 The third step is the restoration of aromaticity when the aryl cation loses a proton (H+) and the product is formed.
- 😀 The sulfonation reaction is reversible, meaning the sulfonic acid group can be removed under specific conditions.
- 😀 Sulfonation can be used to protect reactive groups in a molecule during further chemical reactions.
- 😀 The reaction has practical applications in the synthesis of dyes and pharmaceuticals, including antibiotics like sulfanilamide.
- 😀 The synthesis of sulfanilamide involves sulfonation, and it was one of the first antibiotics used for clinical treatments like meningitis and urinary tract infections.
- 😀 A common example question asks to predict the product of sulfonation when reacting methylbenzene with 2,4-dibromophenol.
Q & A
What is sulfonation in the context of organic chemistry?
-Sulfonation is an electrophilic aromatic substitution reaction where an aromatic compound, such as benzene, reacts with sulfuric acid and sulfur trioxide (SO₃) to form a sulfonated product, like benzene sulfonic acid.
What are the key reactants involved in the sulfonation reaction?
-The key reactants in the sulfonation reaction are sulfuric acid (H₂SO₄) and sulfur trioxide (SO₃), which together generate the electrophile SO₃H+ that reacts with the aromatic compound.
What makes sulfuric acid 'fuming' in the context of sulfonation?
-Sulfuric acid is referred to as 'fuming' because it contains sulfur trioxide (SO₃) in addition to concentrated sulfuric acid, which makes it a highly reactive mixture used in sulfonation reactions.
Why is SO₃H+ considered the electrophile in sulfonation?
-SO₃H+ is the electrophile in sulfonation because it is highly reactive and can attack the electron-rich aromatic ring, initiating the electrophilic aromatic substitution process.
What role does the temperature play in the sulfonation reaction?
-The sulfonation reaction typically requires heating to ensure the efficient formation of the electrophile (SO₃H+), as well as to maintain the reaction conditions that favor the sulfonation process.
How does the sulfonation reaction mechanism proceed after the electrophile is formed?
-After SO₃H+ is formed, it attacks the aromatic ring, creating a positively charged intermediate called the arenium ion. This intermediate then undergoes resonance, which stabilizes the positive charge before the final product is formed.
What is the significance of resonance in the sulfonation mechanism?
-Resonance in the sulfonation mechanism helps stabilize the positively charged arenium ion by delocalizing the positive charge across the aromatic ring, which facilitates the continuation of the reaction and the restoration of aromaticity.
What happens during the aromatization step of sulfonation?
-During aromatization, the arenium ion loses a proton (H+), which restores the aromaticity of the benzene ring, resulting in the formation of the final sulfonated product, such as benzene sulfonic acid.
What are some practical applications of the sulfonation reaction?
-Sulfonation is used in the production of sulfonated drugs, such as sulfanilamide (an antibiotic), and also in the manufacturing of dyes and other industrial chemicals.
Can sulfonation be reversed, and if so, under what conditions?
-Yes, sulfonation is reversible under certain conditions, especially when using dilute sulfuric acid or in the presence of heat, which can break the bonds and reverse the sulfonation process.
Outlines

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنMindmap

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنKeywords

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنHighlights

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنTranscripts

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنتصفح المزيد من مقاطع الفيديو ذات الصلة
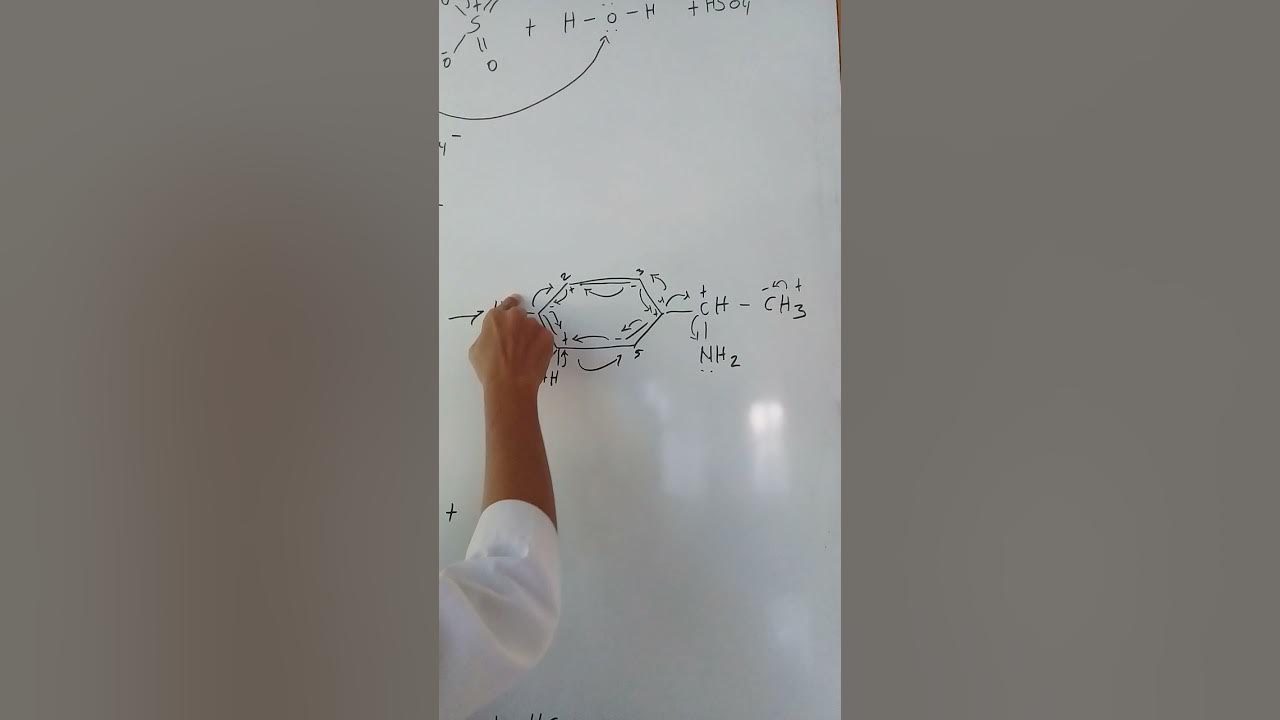
Proses Sulfonasi Senyawa Aromatik
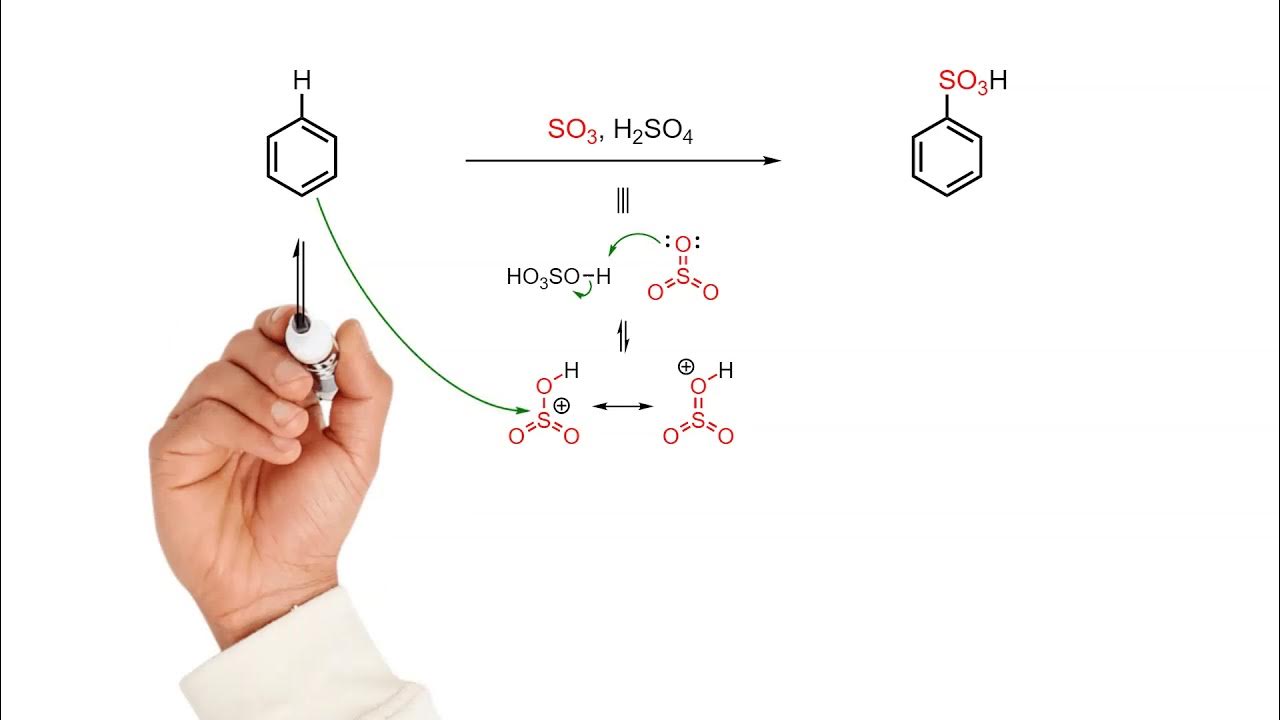
Sulfonation of benzene

Electrophilic Aromatic Substitution

Nucleophilic Aromatic Substitution

Benzene Reaction | Nitration Reaction: Reaction Mechanism, Nitration Method, Nitration Application

Nucleophilic & Electrophilic Aromatic Substitution | Organic Synthesis Chemistry | Retrosynthesis
5.0 / 5 (0 votes)
