Calculating Average Atomic Mass
Summary
TLDRThis tutorial explains how to calculate the average atomic mass of an element using magnesium as an example. It clarifies that the average atomic mass is a weighted average of all natural isotopes' masses by their abundance. The video demonstrates the calculation process by multiplying each isotope's mass with its fractional abundance and summing these products to find the average. It also highlights that the most abundant isotope corresponds to the rounded average atomic mass listed on the periodic table.
Takeaways
- 🔍 The average atomic mass of an element represents the weighted average of the masses of all its natural isotopes by their abundance.
- 📊 The relative atomic mass is also known as the average atomic mass and is found at the bottom of the periodic table entry for each element.
- 🌏 When calculating the average atomic mass, isotopes are not simply averaged; instead, their abundance in nature is taken into account.
- 🧲 Magnesium, with an atomic number of 12, has isotopes with mass numbers 24, 25, and 26, each with different natural abundances.
- 📉 The most abundant isotope of magnesium is magnesium-24, which makes up 78.9% of naturally occurring magnesium.
- 🔢 The mass numbers of isotopes are not whole numbers because atomic mass units are rounded off values based on the masses of protons and neutrons.
- 🧮 To calculate the average atomic mass, multiply the fractional abundance of each isotope by its mass and sum these products.
- 📐 The mass of magnesium-24 is approximately 23.98504, which is very close to its rounded off mass number of 24.
- 📝 The calculated average atomic mass of magnesium (24.3052986), when rounded to two decimal places, matches the value on the periodic table (24.31).
- 🔎 The periodic table's listed average atomic mass can be used to identify the most abundant isotope of an element.
Q & A
What is the average atomic mass of magnesium?
-The average atomic mass of magnesium is 24.31, as indicated on the periodic table.
What is the difference between atomic number and atomic mass number?
-The atomic number of an element is the number of protons in the nucleus, which for magnesium is 12. The atomic mass number is the sum of protons and neutrons, and for magnesium's isotopes, they are 24, 25, and 26 respectively.
What is a weighted average in the context of atomic mass?
-A weighted average in the context of atomic mass refers to the average atomic mass of an element calculated by taking into account the relative abundance of each isotope and their respective masses.
Why is the average atomic mass not a whole number for magnesium?
-The average atomic mass is not a whole number because it is based on the actual masses of the isotopes, which are not whole numbers. These actual masses are rounded off to the nearest whole number for simplicity.
How is the abundance of an isotope determined?
-The abundance of an isotope is determined by its natural occurrence in a sample of the element. For example, magnesium-24 is the most abundant isotope, making up 78.9% of naturally occurring magnesium.
What is the mass of magnesium-24 in atomic mass units?
-The mass of magnesium-24 is approximately 23.98 atomic mass units.
How are the masses of isotopes used to calculate the average atomic mass?
-The masses of isotopes are used to calculate the average atomic mass by multiplying the mass of each isotope by its fractional abundance and then summing these products for all isotopes.
What is the significance of the number 24.31 in the context of magnesium?
-The number 24.31 represents the average atomic mass of magnesium, which is a weighted average that takes into account the natural abundance of its isotopes.
How can you find the most abundant isotope of an element without a table?
-You can find the most abundant isotope of an element without a table by calculating the average atomic mass and rounding it to the nearest whole number, which usually corresponds to the most abundant isotope.
Why is it important to use the fractional form of percent abundance in calculations?
-It is important to use the fractional form of percent abundance in calculations to accurately reflect the proportion of each isotope in the average atomic mass calculation.
What is the relationship between the average atomic mass and the most abundant isotope?
-The most abundant isotope often corresponds to the whole number part of the average atomic mass, as it contributes the greatest portion to the weighted average.
Outlines

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنMindmap

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنKeywords

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنHighlights

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنTranscripts

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنتصفح المزيد من مقاطع الفيديو ذات الصلة
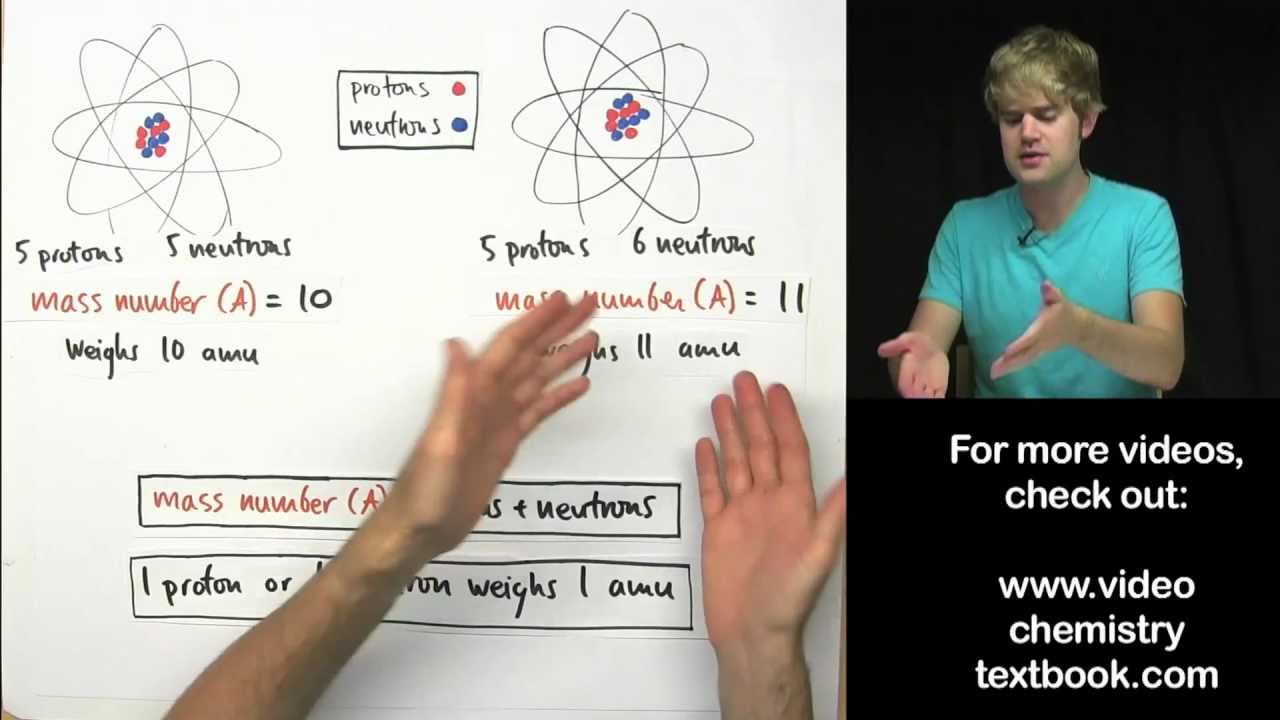
What's the Difference between Mass Number and Atomic Mass?

EDUCA PE| ENSINO MÉDIO | QUÍMICA | 1º ANO I QUANTIDADES EM QUÍMICA I PARTE 1

GCSE Chemistry - Elements, Isotopes & Relative Atomic Mass
A Level Chemistry Revision "Relative Molecular Mass and Relative Formula Mass"
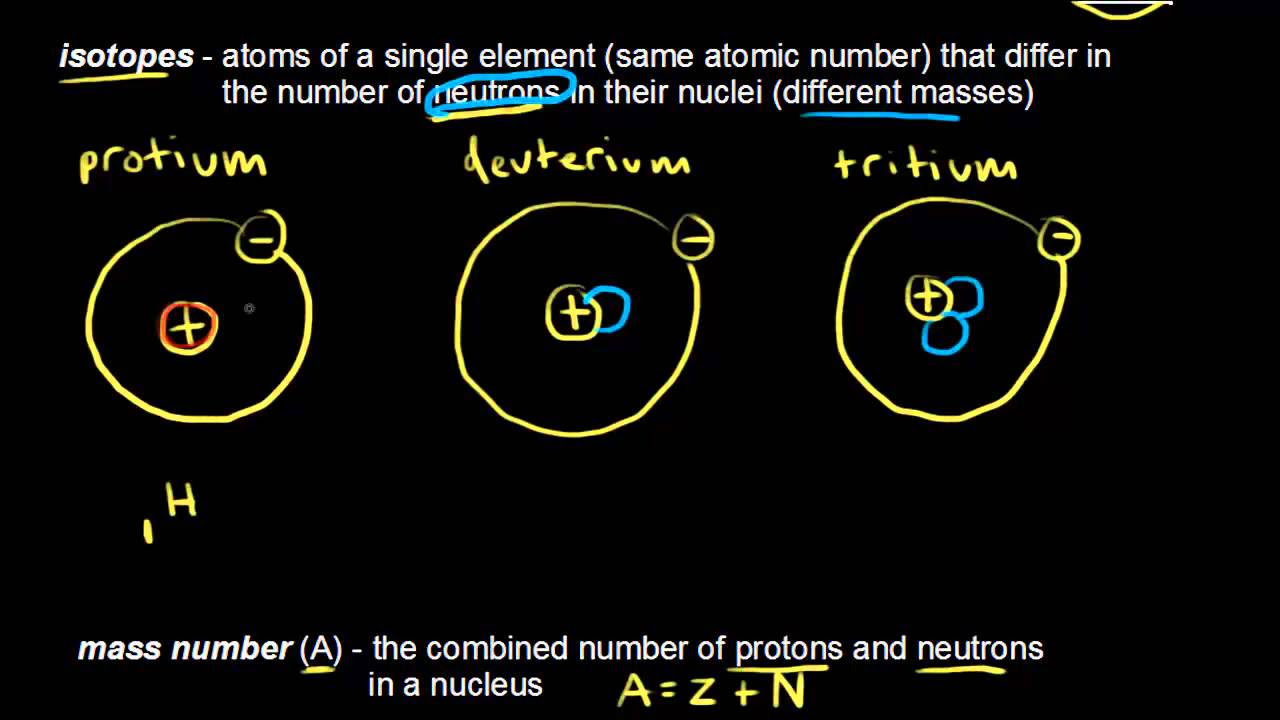
Atomic number, mass number, and isotopes | Chemistry | Khan Academy

CARA MENENTUKAN JUMLAH PROTON, ELEKTRON, NEUTRON | KIMIA SMA KELAS X
5.0 / 5 (0 votes)
