Electrons and Energy Level, Electron Configuration | Grade 9 Science DepEd MELC Quarter 2 Module 1
Summary
TLDRThis video explains how fireworks produce different colors due to the arrangement of electrons in atoms and their emission of light when heated. It introduces Bohr's atomic model, which describes electrons orbiting the nucleus in energy levels, and Schrodinger's quantum mechanical model, which refines this concept using mathematical probabilities. The video also covers electron configuration, including the Aufbau principle, Pauli's exclusion principle, and Hund's rule, to explain how electrons occupy orbitals. The video ends by previewing a future lesson on ionic and covalent bonds.
Takeaways
- 🎆 Fireworks produce different colors due to the arrangement of electrons in atoms.
- 🔥 Metal salts in fireworks emit characteristic colors when heated, with red having the longest wavelength and violet the shortest.
- 🔬 A spectroscope is used to analyze the light emitted by elements, revealing a series of spectral lines that indicate energy levels.
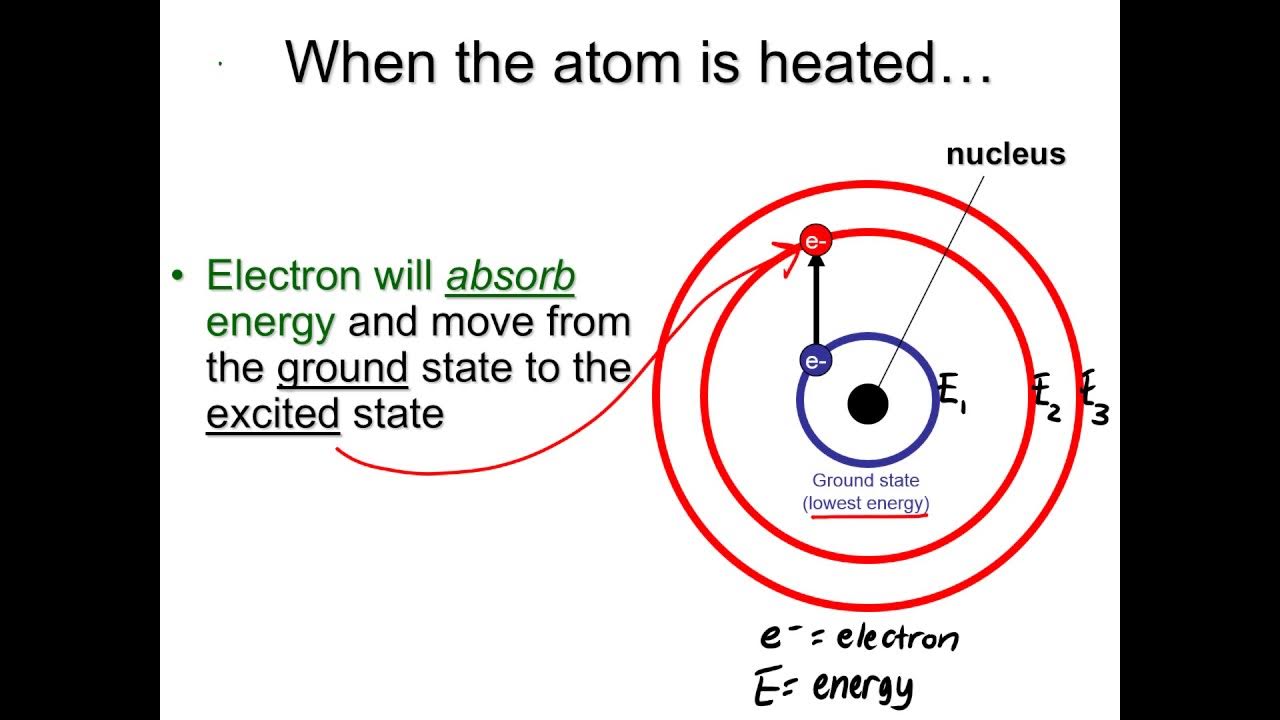
- 🌌 Niels Bohr's atomic model describes electrons orbiting the nucleus in fixed paths, with energy increasing as orbits get farther from the nucleus.
- 🌍 The Bohr model is often referred to as the planetary model, comparing electron movement to planets revolving around the sun.
- ⚛️ Schrodinger and others refined Bohr's model, leading to the quantum mechanical model, which calculates the probability of an electron's location instead of fixed orbits.
- 💡 Electrons reside in energy levels, sub-levels, and atomic orbitals, with orbitals serving as the 'homes' for electrons.
- 🔧 Electron configuration is the most stable arrangement of electrons, with the s, p, d, and f orbitals each holding a specific number of electrons.
- 📜 Three principles guide electron configuration: Aufbau's principle (electrons fill lower energy orbitals first), Pauli's exclusion principle (each orbital holds a maximum of two electrons with opposite spins), and Hund's rule (electrons spread out in orbitals of the same energy before pairing).
- 🚀 Bohr's model works best for single-electron atoms like hydrogen, while the quantum mechanical model applies to more complex atoms.
Q & A
What causes fireworks to display different colors?
-Fireworks emit different colors due to the combustion of metal salts, which release characteristic colors of light when heated. This happens because of the arrangement of electrons in the atoms of the metals.
Why do different colors of light have different wavelengths and energies?
-Each color of light corresponds to a specific wavelength and energy. Red light has the longest wavelength and lowest energy, while violet light has the shortest wavelength and highest energy.
How does heating compounds of different elements produce light?
-When compounds are heated, atoms become excited, and the electrons move to higher energy levels. When these electrons return to their original or lower energy levels, they release light of a specific color.
What does a spectroscope reveal about the light emitted by elements?
-A spectroscope shows the line spectrum of an element, which reveals different energy levels within the atom. The spectral lines correspond to the energy changes occurring in the atom.
What is the Bohr model of the atom?
-Niels Bohr's model describes electrons moving in circular orbits around the nucleus, similar to planets revolving around the sun. Each orbit represents a specific energy level, and the electron's energy increases as its orbit is farther from the nucleus.
What is the quantum mechanical model of the atom?
-The quantum mechanical model, developed by Schrödinger and others, suggests that it is impossible to know the exact path of an electron. Instead, it describes the probability of finding an electron in a certain region of space around the nucleus.
What are atomic orbitals, and how do they relate to electron configuration?
-Atomic orbitals are regions around the nucleus where electrons are most likely to be found. These orbitals are organized into different energy levels and sublevels, and the electron configuration describes the arrangement of electrons in these orbitals.
What are the different shapes of atomic orbitals?
-S orbitals are spherical, P orbitals are dumbbell-shaped, D orbitals have more complex shapes with lobes, and F orbitals have the most diffuse shapes.
What are the three rules used to derive electron configurations?
-The three rules are the Aufbau principle, Pauli's exclusion principle, and Hund's rule. The Aufbau principle states that electrons fill lower energy orbitals first. Pauli's exclusion principle states that a maximum of two electrons can occupy an orbital, and they must have opposite spins. Hund's rule states that electrons spread out to fill available orbitals with the same spin before pairing.
How does electron configuration differ for elements like lithium, sodium, and nitrogen?
-Lithium, with three electrons, has an electron configuration of 1s2 2s1. Sodium, with 11 electrons, has an electron configuration of 1s2 2s2 2p6 3s1. Nitrogen, with seven electrons, spreads its electrons in the P orbital following Hund's rule, with one electron in each P orbital before any pairing.
Outlines

此内容仅限付费用户访问。 请升级后访问。
立即升级Mindmap

此内容仅限付费用户访问。 请升级后访问。
立即升级Keywords

此内容仅限付费用户访问。 请升级后访问。
立即升级Highlights

此内容仅限付费用户访问。 请升级后访问。
立即升级Transcripts

此内容仅限付费用户访问。 请升级后访问。
立即升级5.0 / 5 (0 votes)