The photoelectric and photovoltaic effects | Physics | Khan Academy
Summary
TLDRThis video explains the photoelectric effect, where light striking certain metals ejects electrons. It explores the dependency of the effect on the wavelength and frequency of light, showing that shorter wavelengths produce higher energy electrons. The script highlights the limitations of the wave model and introduces the concept of light as discrete energy packets called photons. It also covers how brightness affects electron emission and the significance of threshold wavelengths for different metals. The video concludes by discussing light's dual wave-particle nature and its applications, such as photovoltaic effects used in solar panels.
Takeaways
- 😀 The photoelectric effect occurs when light shines on certain metals, causing electrons to be ejected.
- 😀 The color (wavelength) of light affects whether the photoelectric effect occurs, with shorter wavelengths like blue being more effective than longer wavelengths like red.
- 😀 Increasing the brightness (intensity) of light increases the number of electrons ejected, but does not affect their energy.
- 😀 The photoelectric effect was puzzling for physicists because the wave model of light could not explain why the effect only occurred with certain colors.
- 😀 Light behaves as both a wave and a particle, with discrete packets of energy called photons explaining the photoelectric effect.
- 😀 The energy of a photon depends on its wavelength, with shorter wavelengths having more energy.
- 😀 Each metal has a threshold wavelength or frequency, below which the photoelectric effect occurs, and above which it does not.
- 😀 The intensity of light in the photon model increases the number of photons, which results in more electrons being ejected, but does not affect the energy of the electrons.
- 😀 If the photon energy is too low (e.g., red light), it cannot eject electrons, even if the light is bright.
- 😀 Light is considered a quantum object, possessing both wave and particle properties, a concept that explains phenomena like diffraction and the photoelectric effect.
- 😀 The photovoltaic effect is similar to the photoelectric effect but involves the creation of voltage instead of ejected electrons, and is used in solar panels to harness energy from light.
Q & A
What is the photoelectric effect?
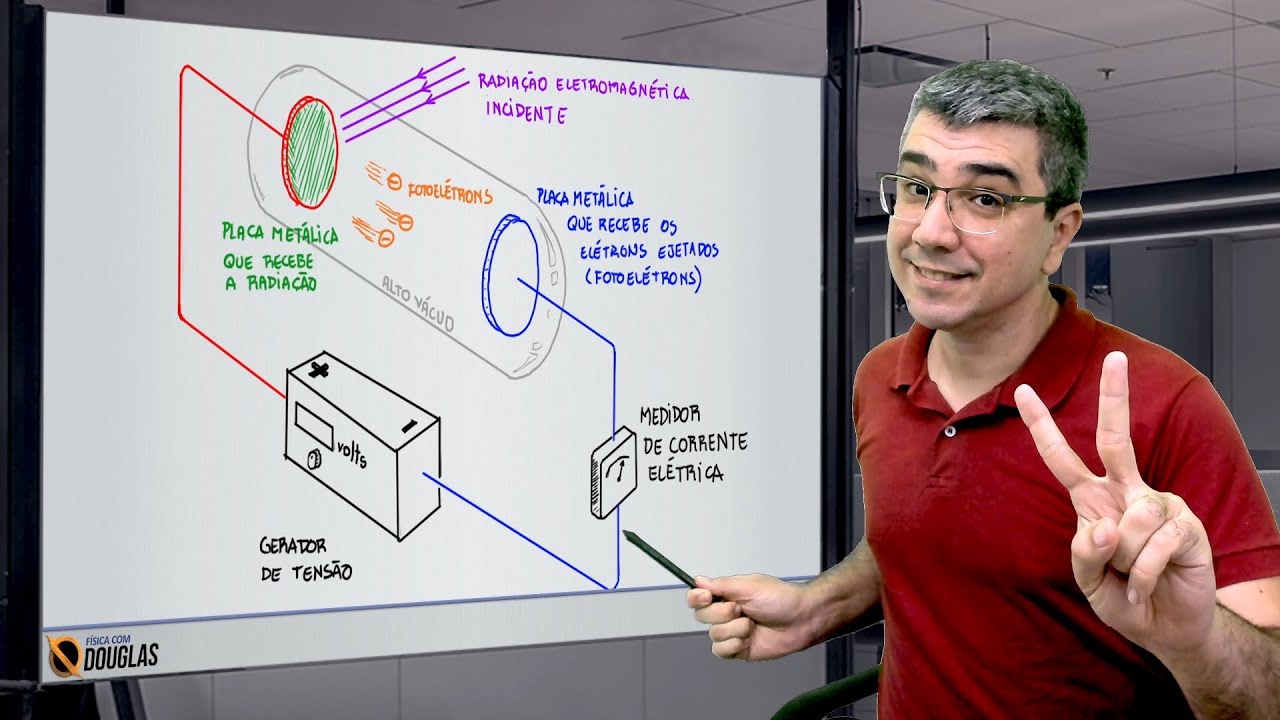
-The photoelectric effect is the phenomenon where electrons are ejected from a metal when light shines on it. The ejected electrons are due to the energy transferred from the light to the electrons.
Why does the photoelectric effect depend on the color (wavelength) of light?
-The photoelectric effect depends on the color of light because the energy of the light's photons, which is related to its wavelength, must be sufficient to free an electron from the metal. If the light's photon energy is too low, electrons will not be ejected.
What happens when red light is shone on potassium in the photoelectric effect?
-When red light is shone on potassium, no photoelectric effect occurs, regardless of the intensity or brightness of the light. This happens because the energy of the photons in red light is too low to eject electrons from potassium.
How does the wavelength of light affect the energy of the ejected electrons?
-As the wavelength of light decreases, the energy of the ejected electrons increases. Shorter wavelengths (like blue light) have more energy per photon, causing the electrons to be ejected with higher kinetic energy.
What role does the intensity of light play in the photoelectric effect?
-The intensity of light affects the number of electrons ejected, but not their energy. Increasing the intensity increases the number of photons, which leads to more electrons being ejected, but the energy of each ejected electron remains the same.
Why does the classical wave model fail to explain the photoelectric effect?
-The classical wave model fails to explain the photoelectric effect because it predicts that any light, if made intense enough, should eject electrons from a metal. However, experiments show that only light below a certain wavelength can cause the effect, regardless of intensity.
What is the new model of light that explains the photoelectric effect?
-The new model of light proposes that light is made up of discrete packets of energy called photons. Each photon carries energy dependent on the light’s frequency, and only photons with enough energy can eject electrons from a metal.
How does the energy of a photon relate to its wavelength?
-The energy of a photon is inversely related to its wavelength: shorter wavelengths (higher frequency) have more energy, while longer wavelengths (lower frequency) have less energy.
What is the threshold wavelength, and how does it relate to the photoelectric effect?
-The threshold wavelength is the maximum wavelength of light that can cause the photoelectric effect in a particular metal. Light with a wavelength longer than this threshold will not cause the effect, regardless of its intensity.
What is the significance of the photovoltaic effect in real-world applications?
-The photovoltaic effect is used in technologies like solar panels. When light strikes a semiconductor material with an inbuilt electric field, electrons are freed but not ejected, allowing them to move and generate a voltage. This principle is used to convert light into electricity in solar cells.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowBrowse More Related Video

Albert Einstein and The Photoelectric Effect | AMS OpenMind

Física moderna: Efeito Fotoelétrico | Física

Physics - Photoelectric effect

Photoelectric Effect, Work Function, Threshold Frequency, Wavelength, Speed & Kinetic Energy, Electr

Dual Nature of Radiation & Matter in 10 mins 😱🔥 Ch 11 Physics Class 12 Boards 2022-23 Score 95+
Física quântica - Efeito fotoelétrico Parte 2 de 2
5.0 / 5 (0 votes)