WAVELENGTHS AND FREQUENCIES OF EM WAVES TAGALOG | GRADE 10 SCIENCE QUARTER 2 MODULE 1 LESSON 3
Summary
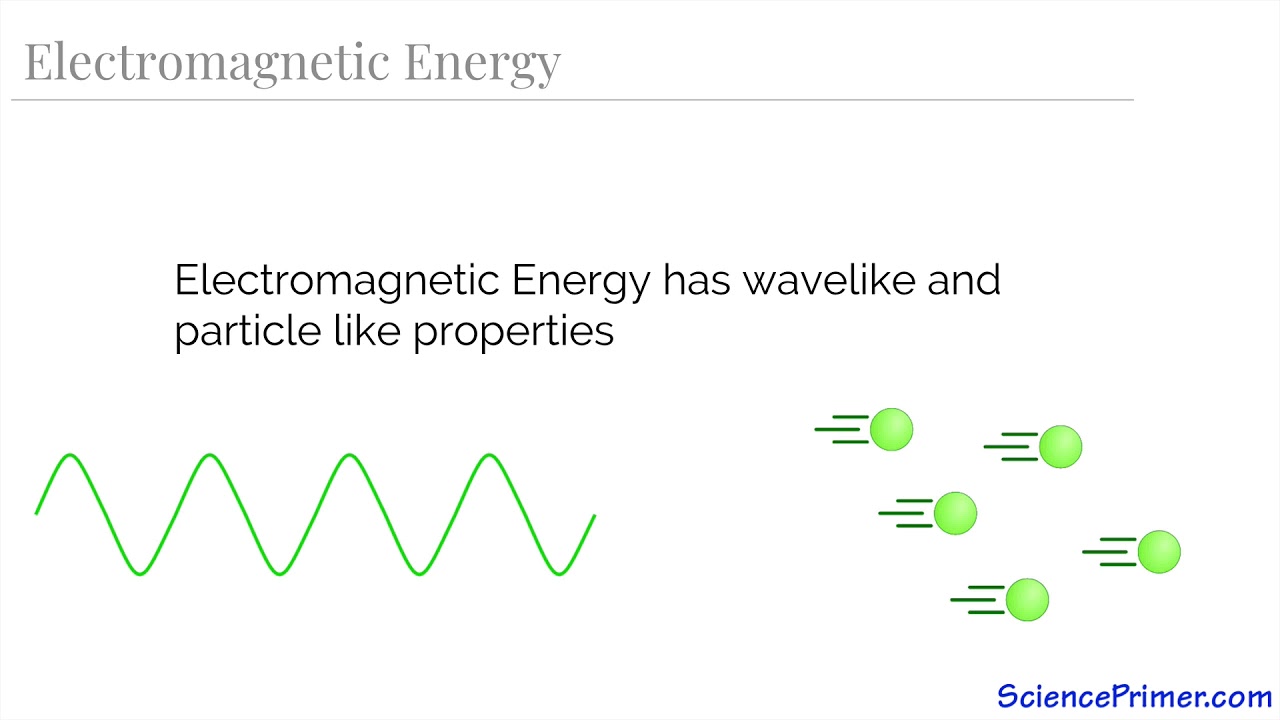
TLDRTeacher Liz's lesson focuses on the relationship between wavelength and frequency of electromagnetic waves, introducing the electromagnetic spectrum's regions and the basic wave equation. The lesson clarifies that as frequency increases, wavelength decreases across the spectrum, from radio waves to gamma rays, affecting photon energy. Practical applications and a pre-test are included to reinforce understanding.
Takeaways
- 🌌 The lesson focuses on understanding the wavelengths and frequencies of electromagnetic waves and their application.
- 🔍 An inverse relationship exists between wavelength and frequency: as one increases, the other decreases.
- 📡 Electromagnetic waves with higher frequencies have shorter wavelengths and vice versa.
- 📶 Radio waves have longer wavelengths compared to ultraviolet rays, which have shorter wavelengths.
- 🌐 All forms of electromagnetic waves travel at the same speed in a vacuum, approximately 3 x 10^8 meters per second.
- ⚡ Gamma rays have the highest frequency and therefore contain photons with the highest energies.
- 🔬 The electromagnetic spectrum is divided into regions based on wavelength and frequency, including radio waves, microwaves, infrared, visible light, ultraviolet, X-rays, and gamma rays.
- 🔑 The basic wave equation is v = fλ, where v is the speed of the wave, f is the frequency, and λ is the wavelength.
- 📚 The mnemonic 'Rabbits Marry In Very Unusual Expensive Gardens' can be used to remember the order of the electromagnetic spectrum from low to high frequency.
- 🔄 When electromagnetic waves pass through different media, their speed and wavelength change, but their frequency remains constant.
- 📉 As frequency increases, the wavelength of an electromagnetic wave decreases.
Q & A
What is the relationship between wavelength and frequency of a wave?
-Wavelength and frequency are inversely proportional to each other. As frequency increases, wavelength decreases, and vice versa.
What happens to wavelengths as frequencies increase on the electromagnetic spectrum?
-As frequencies increase on the electromagnetic spectrum, wavelengths decrease.
Do all forms of electromagnetic waves have the same speed in a vacuum?
-No, all forms of electromagnetic waves travel at the speed of light in a vacuum, which is approximately 3 x 10^8 meters per second.
Which type of electromagnetic waves have the shortest wavelengths?
-Gamma rays have the shortest wavelengths among electromagnetic waves.
What is the electromagnetic spectrum?
-The electromagnetic spectrum is a range of electromagnetic waves with different frequencies and wavelengths.
What is the basic wave equation?
-The basic wave equation is v = fλ, where v is the wave speed, f is the frequency, and λ (lambda) is the wavelength.
How does the speed of light in a vacuum relate to the wavelength and frequency of electromagnetic waves?
-The speed of light in a vacuum (c) is constant and equal to approximately 3 x 10^8 meters per second. The relationship between speed, frequency, and wavelength is given by the equation c = fλ.
What is the significance of the mnemonic device 'Rabbits Marry In Very Unusual Expensive Gardens' in the context of electromagnetic waves?
-The mnemonic device 'Rabbits Marry In Very Unusual Expensive Gardens' helps to remember the order of the electromagnetic spectrum from longest to shortest wavelengths: Radio waves, Microwaves, Infrared, Visible light, Ultraviolet, X-rays, Gamma rays.
How does the refraction of electromagnetic waves affect their speed, wavelength, and frequency?
-When electromagnetic waves encounter a medium, their speed decreases, which causes their wavelength to decrease as well. However, their frequency remains constant.
What is the frequency of a microwave with a wavelength of 1.5 x 10^2 meters?
-To find the frequency, use the wave equation f = v / λ. Given the speed of light (v) is 3 x 10^8 meters per second and the wavelength (λ) is 1.5 x 10^2 meters, the frequency (f) would be approximately 2 x 10^6 Hz.
How does the energy of photons relate to the frequency of electromagnetic waves?
-The energy of photons is directly proportional to the frequency of electromagnetic waves. Higher frequency waves carry photons with higher energy.
Outlines

此内容仅限付费用户访问。 请升级后访问。
立即升级Mindmap

此内容仅限付费用户访问。 请升级后访问。
立即升级Keywords

此内容仅限付费用户访问。 请升级后访问。
立即升级Highlights

此内容仅限付费用户访问。 请升级后访问。
立即升级Transcripts

此内容仅限付费用户访问。 请升级后访问。
立即升级5.0 / 5 (0 votes)