Vapor Pressure | Raoult's Law | Solution Class 12
Summary
TLDRThis video explains key concepts related to vapor pressure and Raoult's law, starting with the distinction between volatile and non-volatile substances. It covers evaporation, vapor pressure in closed containers, and the equilibrium between evaporation and condensation. The video delves into how temperature and intermolecular forces influence vapor pressure. It introduces Raoult's law, describing how partial vapor pressure is directly proportional to mole fractions in a solution. The video concludes with applications of Raoult's law, solving problems on total vapor pressure using Dalton's law, and includes numerical examples for clarity.
Takeaways
- 🌡️ Volatile substances, such as water, gasoline, and ethanol, evaporate at temperatures above or below room temperature, whereas non-volatile substances like salt and sugar do not.
- 💧 Evaporation is the process where surface molecules of a liquid, which have higher kinetic energy, escape and become vapor.
- 🔄 The difference between evaporation and boiling is that boiling occurs at a fixed temperature, while evaporation can happen at any temperature.
- 💭 Vapor pressure is defined as the pressure exerted by vapors on the surface of a liquid in a closed container and is a result of evaporation within that container.
- 🔆 Temperature has a direct relationship with vapor pressure; as temperature increases, so does vapor pressure.
- 🔗 The nature of the liquid and its intermolecular forces inversely affect vapor pressure; liquids with weaker intermolecular forces have higher vapor pressures.
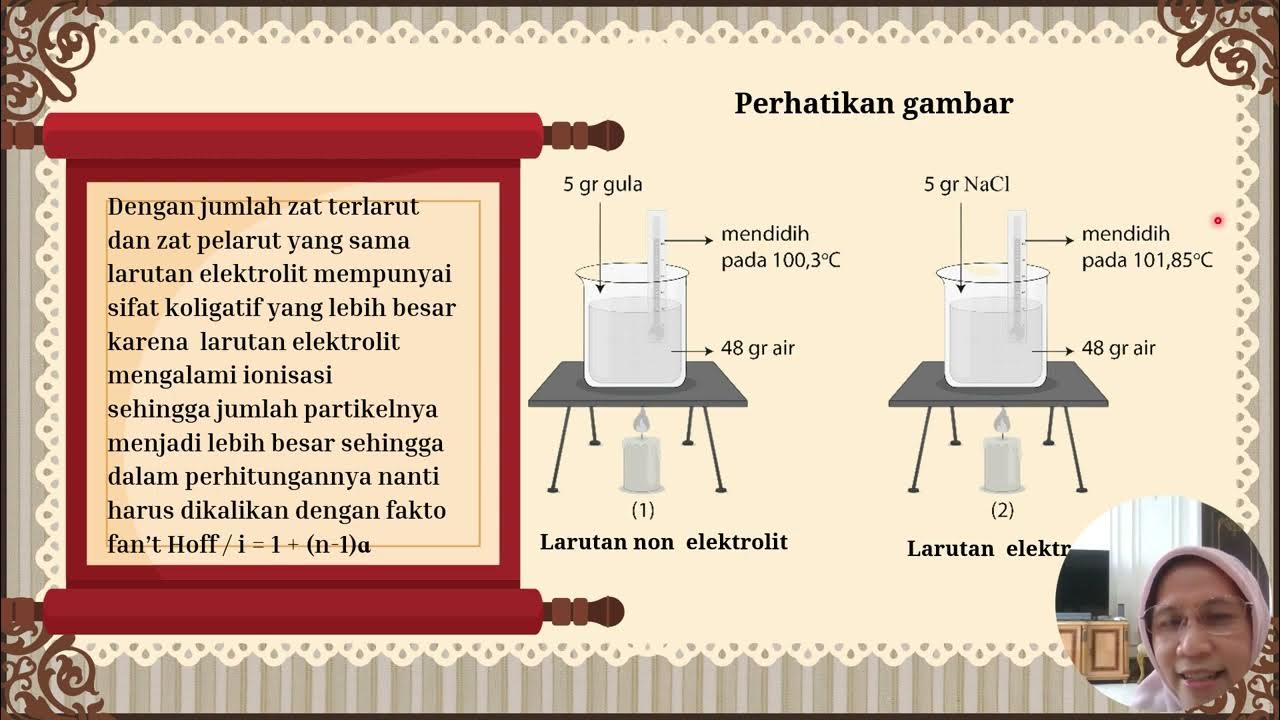
- 🌀 Raoult's Law states that the partial vapor pressure of a liquid in a solution is equal to the vapor pressure of the pure liquid multiplied by its mole fraction in the solution.
- 📉 Dalton's Law is used to calculate the total vapor pressure of a solution, which is the sum of the partial vapor pressures of each volatile component in the solution.
- 📊 Graphically, Raoult's Law can be represented by a straight line where the vapor pressure of a liquid is directly proportional to its mole fraction.
- 🧪 Numerical problems involving Raoult's Law involve calculating the partial vapor pressure of solvents in solutions using their mole fractions and pure vapor pressures.
Q & A
What is a volatile substance?
-A volatile substance is one that evaporates into a gas at room temperature or higher. Examples include water, gasoline, and ethanol.
What is the difference between volatile and non-volatile substances?
-Volatile substances evaporate at room temperature or below, such as water and ethanol, while non-volatile substances, like salt and sugar, do not evaporate at these conditions.
What is evaporation?
-Evaporation is the process by which a liquid transforms into vapor. It occurs when surface molecules with high kinetic energy escape from the liquid into the air.
How is evaporation different from boiling?
-Boiling occurs at a fixed temperature (e.g., 100°C for water), while evaporation can occur at any temperature, even as low as 0°C.
What is vapor pressure?
-Vapor pressure is the pressure exerted by the vapors of a volatile liquid on the surface of the liquid when in a closed container at a given temperature.
How does temperature affect vapor pressure?
-There is a direct relationship between temperature and vapor pressure. As temperature increases, vapor pressure also increases, such as water at 60°C having a higher vapor pressure than at 40°C.
What are the factors affecting vapor pressure?
-Two main factors affect vapor pressure: temperature (direct relationship) and the nature of the liquid (inverse relationship with intermolecular forces).
What is Raoult’s Law?
-Raoult’s Law states that the partial vapor pressure of a liquid in a solution is directly proportional to the mole fraction of that liquid in the solution.
How do you calculate total vapor pressure in a solution?
-Total vapor pressure of a solution can be found using Dalton’s Law, which states that it is the sum of the partial vapor pressures of all the volatile components in the solution.
What is the difference between Raoult's Law and Dalton's Law?
-Raoult’s Law explains the partial vapor pressure of a single volatile component in a solution, while Dalton’s Law is used to find the total vapor pressure of a solution by summing all partial pressures.
Outlines

此内容仅限付费用户访问。 请升级后访问。
立即升级Mindmap

此内容仅限付费用户访问。 请升级后访问。
立即升级Keywords

此内容仅限付费用户访问。 请升级后访问。
立即升级Highlights

此内容仅限付费用户访问。 请升级后访问。
立即升级Transcripts

此内容仅限付费用户访问。 请升级后访问。
立即升级5.0 / 5 (0 votes)