Hybridization of Atomic Orbitals - Sigma & Pi Bonds - Sp Sp2 Sp3
Summary
TLDRThis educational video delves into the concept of atomic orbital hybridization, explaining how atomic orbitals combine to form hybrid orbitals like sp3, sp2, and sp. It details the probability distribution of electrons within orbitals, influenced by the Heisenberg Uncertainty Principle. The video explores carbon's electron configuration and how it forms sp3, sp2, and sp hybrid orbitals, emphasizing their energy levels relative to s and p orbitals. It also discusses degenerate orbitals, the formation of sigma and pi bonds, and the relative strengths of single, double, and triple bonds, providing a foundational understanding of chemical bonding.
Takeaways
- 🌐 Hybridization is the process of combining atomic orbitals to form hybrid orbitals, such as sp3, sp2, and sp, which are blends of s and p orbitals.
- 🔬 The sp3 hybrid orbital is a combination of one s orbital and three p orbitals, resulting in four equivalent orbitals with 25% s character and 75% p character.
- 📊 The sp2 hybrid orbital is formed by combining one s orbital and two p orbitals, creating three equivalent orbitals with approximately 33% s character and 67% p character.
- 📈 The sp hybrid orbital is a mix of one s orbital and one p orbital, leading to two equivalent orbitals with 50% s character and 50% p character.
- 📍 Hybrid orbitals are positioned at energy levels that reflect their composition, with more p character placing them closer to the p orbitals' energy level.
- 🌀 Degenerate orbitals, like the sp3, sp2, and sp hybrids, have the same energy and are used to place electrons one at a time with parallel spins.
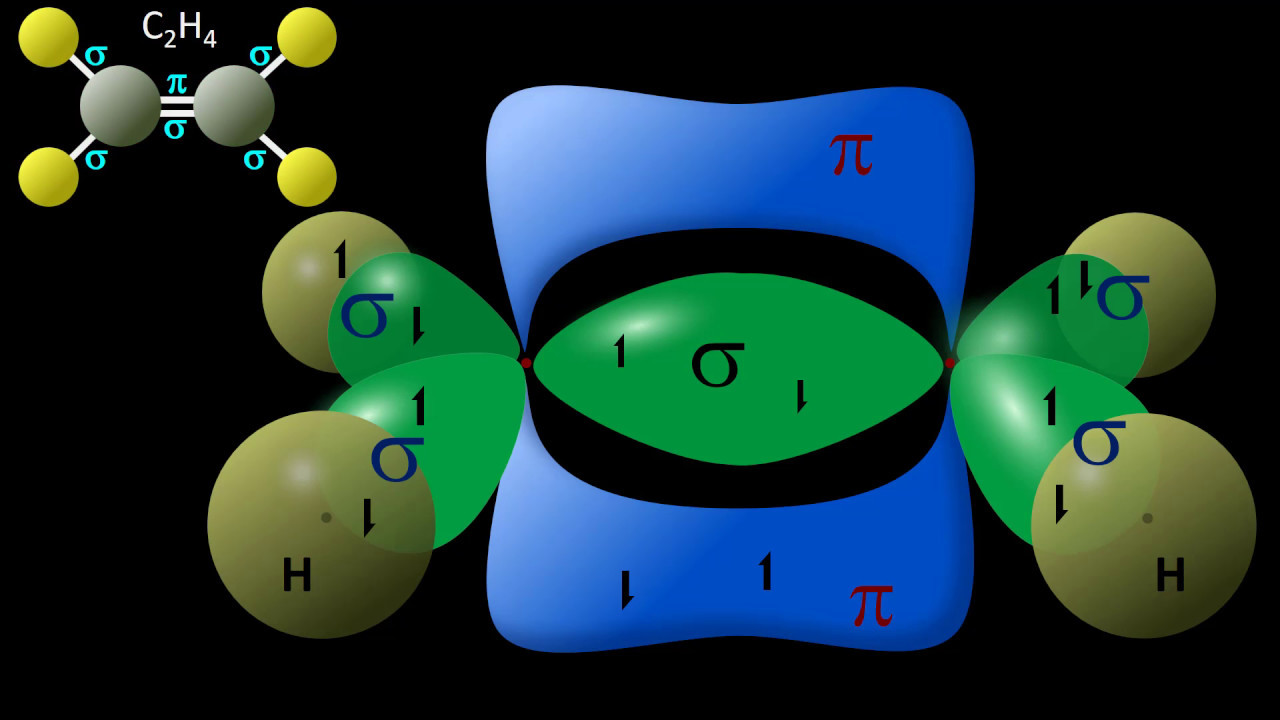
- 🔗 Sigma bonds are formed from the overlap of hybrid orbitals and are present in single, double, and triple bonds, with triple bonds being the strongest.
- 🔄 Unhybridized p orbitals are used to form pi bonds, which are weaker than sigma bonds and are found in double and triple bonds alongside sigma bonds.
- 🔢 The number of sigma bonds in a molecule can be counted as one for each bond, while pi bonds are counted as one for each double bond and two for each triple bond.
- 📚 Understanding hybridization and bond types is crucial for predicting molecular geometry and the strength of chemical bonds.
Q & A
What is the definition of hybridization in atomic orbitals?
-Hybridization is the process of combining atomic orbitals to form hybrid orbitals. It involves mixing different types of orbitals, such as s, p, and d orbitals, to create new orbitals that are suitable for bonding.
What are the different types of hybrid orbitals mentioned in the script?
-The script mentions sp3, sp2, and sp hybrid orbitals. These are created by combining one s orbital with three p orbitals, one s orbital with two p orbitals, and one s orbital with one p orbital, respectively.
How does the energy level of hybrid orbitals compare to the original atomic orbitals?
-Hybrid orbitals generally have an energy level that is closer to the p orbitals than the s orbitals due to the higher proportion of p character in the hybrid orbitals. The energy level also depends on the number of p orbitals involved in the hybridization.
What is the significance of the term 'degenerate orbitals' in the context of hybridization?
-Degenerate orbitals are orbitals that have the same energy. In the context of hybridization, all the sp3, sp2, or sp hybrid orbitals formed are degenerate, meaning they have the same energy level.
How does the electron configuration of carbon relate to its hybridization?
-Carbon has an electron configuration of 1s2 2s2 2p2, with four valence electrons. When carbon forms sp3 hybrid orbitals, it uses all four orbitals (one s and three p orbitals) to create four sp3 hybrid orbitals.
Why is it important to add electrons to degenerate orbitals one at a time with parallel spins?
-Adding electrons one at a time with parallel spins to degenerate orbitals maximizes the stability of the atom by minimizing electron-electron repulsion. This follows Hund's rule, which states that electrons will fill orbitals in a way that maximizes the number of unpaired electrons in degenerate orbitals.
What is the percentage of s and p character in an sp3 hybrid orbital?
-An sp3 hybrid orbital has 25% s character and 75% p character, as it is formed by combining one s orbital with three p orbitals.
How many unhybridized p orbitals are left after forming sp2 hybrid orbitals?
-After forming sp2 hybrid orbitals, one p orbital remains unhybridized because only two of the three p orbitals are used in the hybridization process.
What is the difference between sigma and pi bonds in terms of strength?
-Sigma bonds are stronger than pi bonds. While a triple bond is stronger than a single bond due to the presence of three bonds (one sigma and two pi), when comparing individual bond types, a sigma bond is more difficult to break than a pi bond.
How can you determine the number of sigma and pi bonds in a molecular structure?
-In a molecular structure, every single bond contains one sigma bond, and every double bond contains one sigma and one pi bond. A triple bond contains one sigma and two pi bonds. By counting the number of single, double, and triple bonds, you can determine the total number of sigma and pi bonds.
Outlines

此内容仅限付费用户访问。 请升级后访问。
立即升级Mindmap

此内容仅限付费用户访问。 请升级后访问。
立即升级Keywords

此内容仅限付费用户访问。 请升级后访问。
立即升级Highlights

此内容仅限付费用户访问。 请升级后访问。
立即升级Transcripts

此内容仅限付费用户访问。 请升级后访问。
立即升级浏览更多相关视频

Hybridation des orbitales atomiques (1) - Intro & sp3

MENENTUKAN BENTUK MOLEKUL : TEORI HIBRIDISASI (KIMIA SMA KELAS 10)
Hybridization

Valence Bond Theory & Hybrid Atomic Orbitals

An Introduction to Inorganic Chemistry- Lecture 2
Hybrid Orbitals explained - Valence Bond Theory | Orbital Hybridization sp3 sp2 sp
5.0 / 5 (0 votes)
