Hybridization
Summary
TLDRThe script explains the concept of hybridization in atomic orbitals, drawing an analogy with hybrid cars and bikes. It uses carbon tetrachloride and ammonia as examples to illustrate how atomic orbitals hybridize to form new, equal-energy orbitals (sp3) for bonding. The script also differentiates between sigma and pi bonds, explaining that sigma bonds are hybridized while pi bonds are not, and applies this to molecules like carbon dioxide and ozone.
Takeaways
- 🚗 Hybridization in chemistry is compared to hybrid cars or bikes, combining two different things to create something new.
- 🌀 The process of hybridization involves mixing atomic orbitals to form new, identical orbitals of equal energy.
- 🔍 Carbon tetrachloride is used as an example to explain why hybridization is necessary, ensuring all bonds are equal in energy.
- 🔬 In carbon tetrachloride, carbon's 2s and three 2p orbitals hybridize to form four sp3 orbitals, accommodating four chlorine atoms.
- 💡 Hybridization is necessary for single bonds to ensure they are of equal energy, now referred to as sigma bonds.
- 🌐 Lone pairs of electrons also undergo hybridization, as seen in ammonia, where nitrogen's five valence electrons hybridize into four sp3 orbitals.
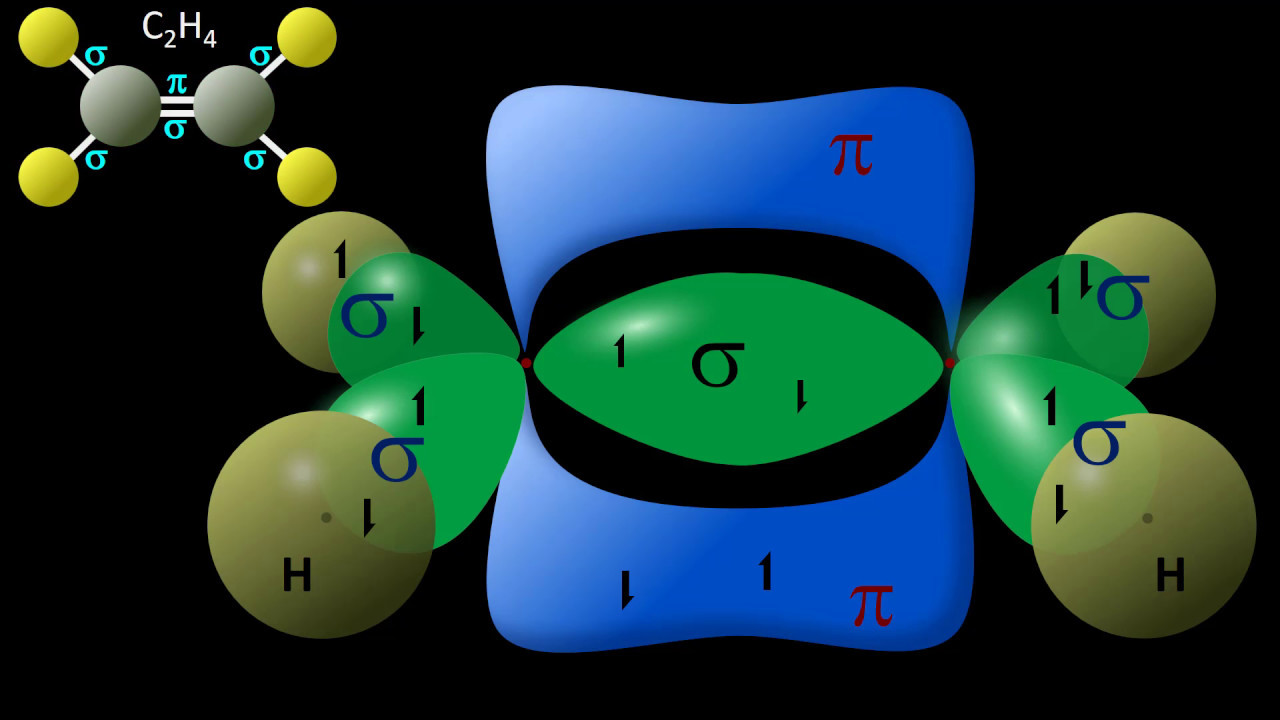
- 🔗 In multiple bonds, one bond is a sigma bond (hybridized) and the others are pi bonds (not hybridized, just p orbitals overlapping).
- 🌄 Carbon dioxide is used to illustrate hybridization in a double bond, with one sigma and one pi bond, leading to sp hybridization for carbon.
- 🎯 Oxygen in carbon dioxide shows sp2 hybridization, with one sigma bond and two lone pairs hybridized, leaving one pi bond as a p orbital.
- 🔄 Hybridization varies with different molecules; for example, ozone (O3) has different hybridizations for each oxygen atom due to varying numbers of sigma bonds and lone pairs.
Q & A
What is the concept of hybridization in atomic orbitals?
-Hybridization is the process where two or more atomic orbitals combine to form a new set of identical orbitals, known as hybrid orbitals. This process occurs to create new orbitals that are equal in energy, allowing for the formation of molecules with specific bonding characteristics.
Why is hybridization necessary in chemistry?
-Hybridization is necessary to explain the bonding in molecules where the electron domains around a central atom are not all equivalent, such as in carbon tetrachloride where a carbon atom is bonded to four chlorine atoms with equal bond energies.
How does hybridization ensure equal bond energies in carbon tetrachloride?
-In carbon tetrachloride, the carbon atom hybridizes its 2s and three 2p orbitals to form four sp3 hybrid orbitals, each of which is equal in energy. This allows for four equal bonds to be formed with the four chlorine atoms.
What are the different types of bonds formed in hybridized orbitals?
-Hybridized orbitals can form sigma (σ) bonds, which are the first bond in a multiple bond and involve head-on overlapping of orbitals. Pi (π) bonds are the additional bonds in a multiple bond and involve side-by-side overlapping of p orbitals.
How does the hybridization of nitrogen in ammonia differ from that in carbon tetrachloride?
-In ammonia, the nitrogen atom has five valence electrons, including three that form bonds with hydrogen atoms and two that are lone pairs. This results in sp3 hybridization with one s and three p orbitals mixing to form four equivalent hybrid orbitals.
What is the difference between sigma and pi bonds?
-Sigma bonds are the first bond formed in a covalent bond and are formed by the end-to-end overlap of hybridized orbitals. Pi bonds are weaker and are formed by the side-to-side overlap of p orbitals, and they occur in multiple bonds.
How does the hybridization of carbon in carbon dioxide differ from that in carbon tetrachloride?
-In carbon dioxide, the carbon atom forms a double bond with oxygen, resulting in sp hybridization with one s and one p orbital mixing to form two equivalent hybrid orbitals, one for the sigma bond and one for the pi bond.
What is the hybridization of oxygen in carbon dioxide?
-Oxygen in carbon dioxide has two sigma bonds (one with carbon and one with another oxygen) and two lone pairs, resulting in sp2 hybridization with one s and two p orbitals mixing to form three equivalent hybrid orbitals.
How does the hybridization of atoms in a triple bond differ from that in a double bond?
-In a triple bond, one sigma bond and two pi bonds are formed. The sigma bond is hybridized, while the two pi bonds are not and consist of pure p orbitals. This is different from a double bond, where only one sigma bond is hybridized and one pi bond is formed from p orbitals.
What is the hybridization of nitrogen in a nitrogen molecule (N2)?
-In a nitrogen molecule, each nitrogen atom forms a triple bond with the other, resulting in sp hybridization with one s and one p orbital mixing to form two equivalent hybrid orbitals, one for the sigma bond and one for each of the two pi bonds.
How does the hybridization of atoms in ozone (O3) differ at each oxygen atom?
-In ozone, the central oxygen atom has sp2 hybridization with one sigma bond and three lone pairs, while the terminal oxygen atoms have sp2 hybridization with one sigma bond, one lone pair, and one pi bond.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowBrowse More Related Video

Valence Bond Theory & Hybrid Atomic Orbitals

Hybridization of Atomic Orbitals - Sigma & Pi Bonds - Sp Sp2 Sp3

Hybridation des orbitales atomiques (1) - Intro & sp3

MENENTUKAN BENTUK MOLEKUL : TEORI HIBRIDISASI (KIMIA SMA KELAS 10)
Hybrid Orbitals explained - Valence Bond Theory | Orbital Hybridization sp3 sp2 sp

Draw the Orbital Overlap Diagram of O2 (Oxygen gas)
5.0 / 5 (0 votes)