An Introduction to Inorganic Chemistry- Lecture 2
Summary
TLDRThis lecture introduces molecular orbital theory, explaining how atomic orbitals combine to form molecular orbitals in molecules. It begins with a review of atomic orbitals and electron sharing in molecules, then delves into the formation of bonding and anti-bonding orbitals. The lecture covers how electrons fill these molecular orbitals and the energy level diagrams for simple diatomic molecules like hydrogen, oxygen, and nitrogen. The model helps predict molecular behavior and bonding strength. Though not examined in the course, this theory is essential for understanding molecular structure and reactivity in chemistry.
Takeaways
- 😀 **Molecular Orbital Theory (MO Theory)** explains the formation of bonds through the overlap of atomic orbitals to create molecular orbitals.
- 😀 **Bonding and anti-bonding orbitals**: Bonding orbitals lower the molecule's energy, stabilizing it, while anti-bonding orbitals destabilize it.
- 😀 **Linear Combination of Atomic Orbitals (LCAO)** is used to combine atomic orbitals, forming molecular orbitals that describe the molecule's electron configuration.
- 😀 **Electron configuration** in molecular orbitals follows the **Pauli Exclusion Principle**, with each orbital holding up to two electrons with opposite spins.
- 😀 **Bond order** is calculated by subtracting the number of electrons in anti-bonding orbitals from those in bonding orbitals, indicating bond strength.
- 😀 For molecules like **H₂**, the bond order is 1, resulting in a stable bond, while lower bond orders, like in **Li₂**, indicate weaker bonds.
- 😀 **HOMO (Highest Occupied Molecular Orbital)** and **LUMO (Lowest Unoccupied Molecular Orbital)** are critical for understanding molecular reactivity and chemical behavior.
- 😀 The lecture covered simple **diatomic molecules** (e.g., H₂, O₂) and discussed the variation in molecular orbital diagrams as you move across the periodic table.
- 😀 In **oxygen (O₂)** and **nitrogen (N₂)**, the overlap of s and p orbitals results in a mix of bonding and anti-bonding orbitals, influencing their stability.
- 😀 The theory emphasizes the need for a qualitative understanding of bonding, as the mathematics behind molecular orbitals can be complex but are important for explaining molecular structure.
Q & A
What is the main focus of the lecture regarding atomic orbitals?
-The main focus is to explain how atomic orbitals interact and combine to form molecular orbitals, which ultimately affects the bonding in molecules. This is the foundation for understanding the structure and behavior of molecules.
How does Molecular Orbital Theory differ from Lewis structures?
-Molecular Orbital Theory goes beyond Lewis structures by considering the actual overlap of atomic orbitals to form bonding and anti-bonding molecular orbitals, whereas Lewis structures are more about the arrangement of atoms and electrons in a molecule without considering orbital interactions.
What happens when atomic orbitals overlap in Molecular Orbital Theory?
-When atomic orbitals overlap, they combine to form two types of molecular orbitals: a bonding orbital (lower energy) and an anti-bonding orbital (higher energy). This overlap determines the stability and strength of the bond in the molecule.
What is the significance of the Pauli Exclusion Principle in Molecular Orbital Theory?
-The Pauli Exclusion Principle dictates that no more than two electrons can occupy a single molecular orbital, and they must have opposite spins. This principle is essential for determining the electron configurations in molecular orbitals.
How do energy level diagrams help explain molecular bonding?
-Energy level diagrams visually represent the relative energies of bonding and anti-bonding molecular orbitals. These diagrams help predict how electrons will fill the molecular orbitals and, in turn, the stability and properties of the molecule.
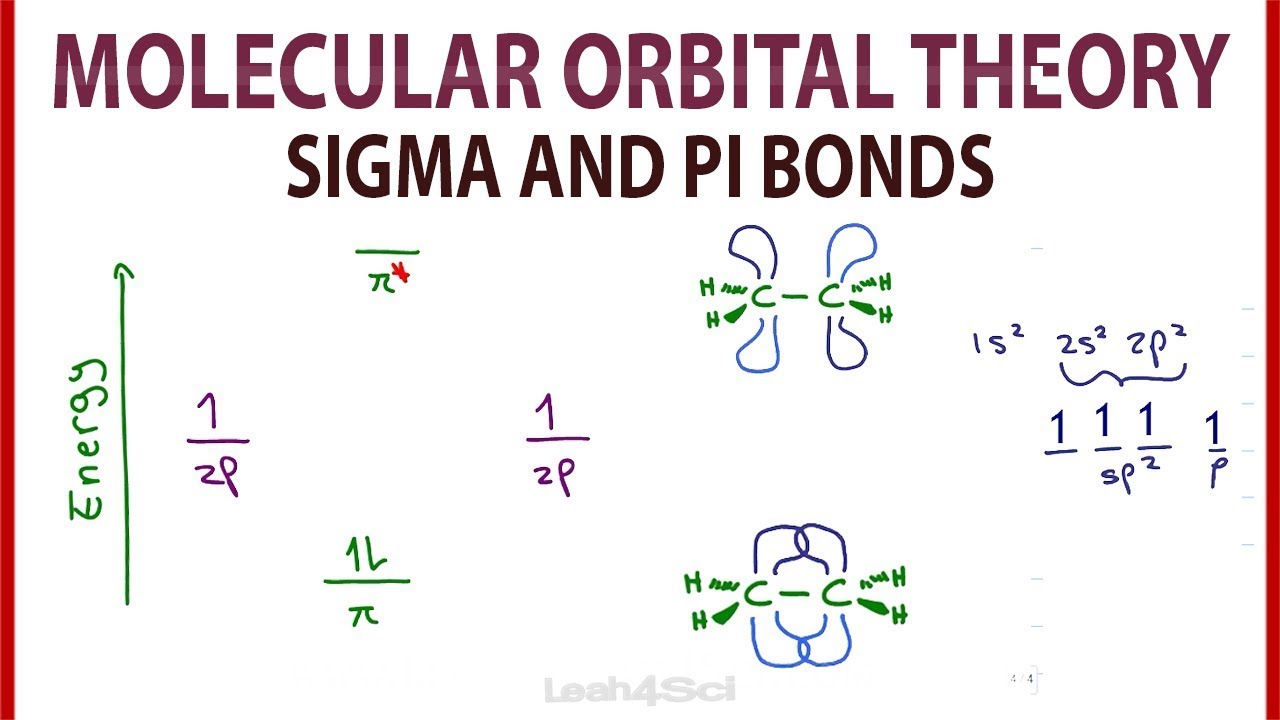
What role do sigma and pi molecular orbitals play in molecular bonding?
-Sigma (σ) and pi (π) molecular orbitals form from the overlap of atomic orbitals. Sigma bonds generally result from head-on overlaps and are stronger, while pi bonds form from side-to-side overlaps and are weaker, often playing a role in double and triple bonds.
How does the molecular orbital theory explain the bonding in hydrogen molecules (H₂)?
-In the hydrogen molecule (H₂), two 1s atomic orbitals overlap to form one bonding molecular orbital (lower energy) and one anti-bonding molecular orbital (higher energy). The two electrons in H₂ occupy the bonding molecular orbital, resulting in a stable bond.
What is the difference in bonding between molecules like nitrogen and oxygen according to the molecular orbital theory?
-In nitrogen, the molecular orbitals follow a standard energy order with sigma and pi orbitals, while in oxygen, the energy levels of the sigma and pi orbitals reverse. This results in different bond strengths and molecular properties for these two molecules.
Why is it important to understand molecular orbital theory, even if it's not directly tested in exams?
-Molecular orbital theory provides a deeper understanding of chemical bonding, electron configurations, and molecular behavior. This foundational knowledge is critical for more advanced topics in chemistry and helps explain real-world chemical phenomena.
What practical applications does molecular orbital theory have beyond the classroom?
-Molecular orbital theory has numerous applications in fields like materials science, drug design, and understanding chemical reactions. By predicting molecular stability, bond strength, and reactivity, it helps in designing new molecules with specific properties.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowBrowse More Related Video

Hybridation des orbitales atomiques (1) - Intro & sp3

11 Chap 4 | Chemical Bonding 10 | Molecular Orbital Theory IIT JEE NEET || MOT Part I Introduction |

Kimia Dasar 1 - Teori Orbital Molekul
Molecular Orbital MO Theory Simplified for Sigma and Pi Bonds

Teoria da Ligação de Valência e Teoria do Orbital Molecular ... Qual a diferença?

Molecular Orbital Theory Boron Trifluoride BF3 | Professor Adam Teaches
5.0 / 5 (0 votes)