Knocking Electrons With Light—The Photoelectric Effect
Summary
TLDRIn this educational video, the presenter explores the photoelectric effect, demonstrating how light, composed of particles called photons, can transfer energy to electrons. Using an electroscope, they show that while visible light cannot dislodge electrons, high-energy ultraviolet light can. The experiment illustrates the dual nature of light as both a wave and a particle, with higher frequency light behaving more like particles. The video concludes with a discussion on the wave-particle duality and the concept of particles as vibrations of quantum fields.
Takeaways
- 🔬 The experiment demonstrates that light is composed of particles, specifically photons, which can be observed through the photoelectric effect.
- 🔋 An electroscope is used to measure the electric charge on a metal plate by detecting the movement of a needle due to the charge.
- 🎈 Rubbing a balloon on hair transfers electrons to the balloon, which can then be transferred to the electroscope plate to charge it.
- 🚫 Even high-intensity visible light (red, green, and blue) does not have enough energy per photon to release electrons from the metal plate.
- 🌞 Shortwave ultraviolet light, also known as UVC, has enough energy per photon to knock electrons off the metal plate, causing the electroscope to discharge.
- ⚡ The brightness of the light affects the rate at which electrons are released but not the ability to release them; it's the energy per photon that matters.
- 🔀 When the plate is positively charged with protons, UV light does not easily release these charges, unlike with extra electrons.
- 💡 Neon light bulbs require a certain voltage to ionize the gas inside and emit light, but UV light can help initiate this process even below the usual voltage.
- 🌌 The concept of wave-particle duality is introduced, explaining how light can exhibit properties of both waves and particles depending on its frequency.
- 📚 The video concludes by highlighting Einstein's Nobel Prize-winning explanation of the photoelectric effect and the importance of understanding light as both a wave and a particle.
Q & A
What is the purpose of the electroscope used in the video?
-The electroscope is used to measure the amount of electric charge on a plate by detecting the movement of a needle due to the presence of charged particles.
How does the presenter charge the electroscope plate with electrons?
-The presenter charges the electroscope plate with electrons by rubbing a balloon on their hair, which transfers electrons from the hair to the balloon, and then wiping the balloon on the plate.
Why does touching the charged plate cause the needle to move back to its original position?
-Touching the charged plate allows the electrons to escape from the plate into the hand, neutralizing the charge and causing the needle to return to its original position.
What is the significance of the photoelectric effect demonstrated in the video?
-The photoelectric effect demonstrates that light can behave as particles, called photons, which have enough energy to knock electrons off a surface when the light's frequency is high enough.
Why doesn't visible light (red, green, and blue) discharge the electroscope plate?
-Visible light, including red, green, and blue, doesn't have enough energy per photon to overcome the binding energy of the electrons on the plate, so it cannot discharge it.
What type of light is successful in discharging the electroscope plate in the video?
-Shortwave ultraviolet light, also known as UVC light, is successful in discharging the electroscope plate because it has a higher energy per photon compared to visible light.
How does the brightness of the light affect the photoelectric effect?
-The brightness or intensity of the light affects the rate at which electrons are knocked off but does not affect the ability to knock them off. Higher intensity light increases the number of photons hitting the surface per second, increasing the rate of electron ejection.
What is the difference between charging the plate with extra electrons versus extra protons?
-Charging the plate with extra electrons results in a negative charge, which can be discharged with UV light. Charging with extra protons results in a positive charge, which is harder to discharge with light because protons are more tightly bound within the atomic nucleus.
Why does the neon light bulb require a certain voltage to light up?
-The neon light bulb requires a certain voltage to strip electrons off the gas inside, which then emit light. Below this voltage threshold, not enough electrons are excited to produce light.
How does the presenter demonstrate the photoelectric effect with the neon light bulb?
-The presenter demonstrates the photoelectric effect by shining shortwave ultraviolet light on the neon bulb, which is not enough to light it up by itself due to low voltage. The UV light provides the necessary energy to excite the gas, allowing the bulb to light up even at a voltage below the normal threshold.
What is the wave-particle duality and how does it relate to light?
-Wave-particle duality is the concept that light and other quantum entities can exhibit both wave-like and particle-like properties. The manifestation as a wave or particle depends on the frequency or wavelength; higher frequencies tend to exhibit particle-like properties (photons), while lower frequencies exhibit wave-like properties.
Outlines

此内容仅限付费用户访问。 请升级后访问。
立即升级Mindmap

此内容仅限付费用户访问。 请升级后访问。
立即升级Keywords

此内容仅限付费用户访问。 请升级后访问。
立即升级Highlights

此内容仅限付费用户访问。 请升级后访问。
立即升级Transcripts

此内容仅限付费用户访问。 请升级后访问。
立即升级浏览更多相关视频
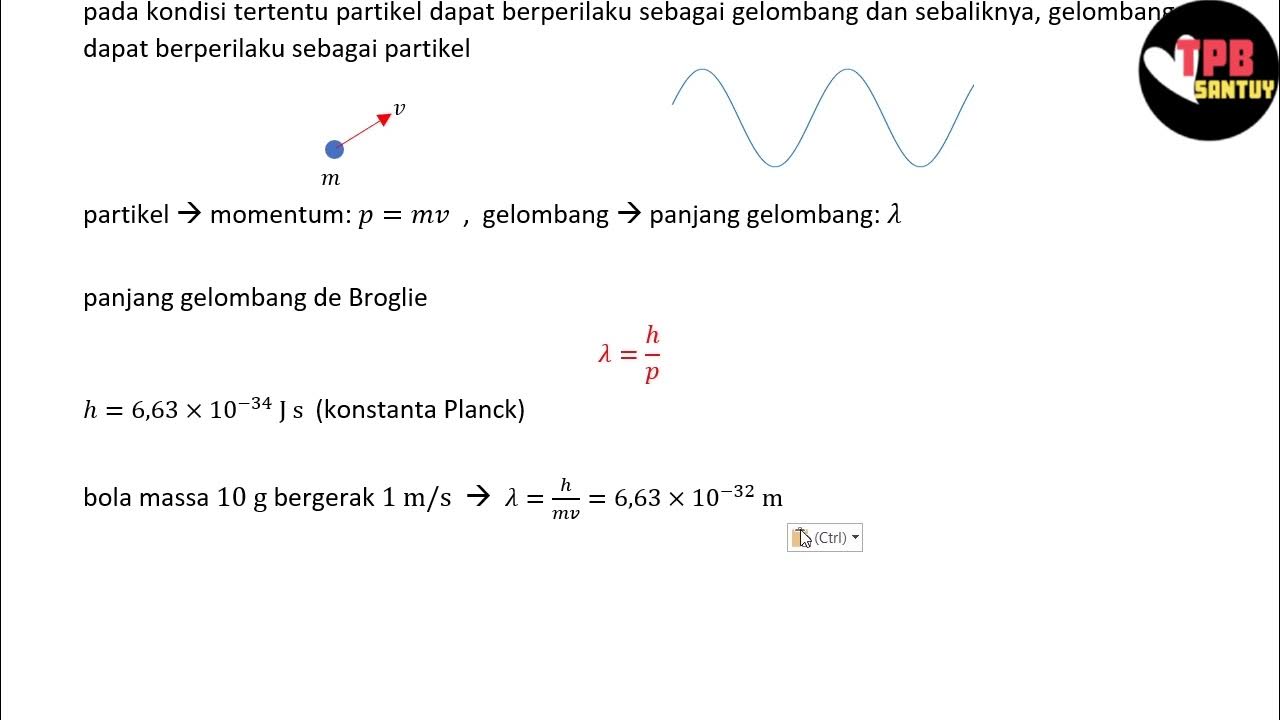
Dualisme Gelombang-Partikel | Fenomena Kuantum | Part 1 | Fisika Dasar

Photoelectric Effect Theory Lesson

3.3.2 - Radiação eletromagnética: Teoria Quântica - Efeito Fotoelétrico (Einstein)

Energy Levels & Emission Spectra - A-level Physics

The photoelectric and photovoltaic effects | Physics | Khan Academy

Physics - Photoelectric effect
5.0 / 5 (0 votes)
