Pure solids & Pure liquids can be ignored while writing expression for equilibrium constant Kc
Summary
TLDRThe script delves into the concept of equilibrium constants, particularly focusing on why the concentrations of pure solids and liquids are omitted in the expression for KC. It clarifies that molarity, which is the basis for KC, is calculated as moles per volume, and since the density of pure substances remains constant regardless of volume changes, their concentration is considered constant. This is why they are ignored in the equilibrium expression. The script uses examples, including ethyl acetate and acetic acid, to illustrate this principle, emphasizing the importance of understanding the behavior of gases in contrast to pure solids and liquids.
Takeaways
- 🧪 The equilibrium constant, KC, is used to express the concentrations of reactants and products at equilibrium in a chemical reaction.
- 📚 Solids and pure liquids are often omitted from the expression for KC because their concentration remains constant due to their intensive properties.
- 🔍 Molarity, or molar concentration, is defined as the number of moles of a substance divided by the volume of the solution.
- 📉 The concentration of pure solids and liquids does not change with volume because their density, an intensive property, remains the same regardless of the amount of substance.
- 🌡️ For gases, the concentration can vary with changes in volume and mass, which is why they are included in the expression for KC.
- 🧩 In the given example, copper oxide and copper nitrate are ignored in the KC expression because they are solids with constant molar concentration.
- 🌌 The concentration of gases like NO2 and O2 is considered in the KC expression because they can change with volume and mass.
- 🛑 The script emphasizes that the concentration of pure liquids, such as water in the example of ethyl acetate and acetic acid, is constant and thus not included in the KC expression.
- 🔄 The equilibrium constant is important for understanding the extent to which a reaction proceeds and the relative concentrations of products and reactants at equilibrium.
- 📈 The value of KC can be used to predict the direction a reaction will take and the position of equilibrium for a given set of conditions.
- 🌟 Understanding the principles behind the inclusion or exclusion of substances in the KC expression is crucial for correctly applying chemical equilibrium concepts.
Q & A
Why are solids and pure liquids typically not included in the expression for the equilibrium constant (KC)?
-Solids and pure liquids are not included in the KC expression because their concentration remains constant. This is due to their density being an intensive property, meaning it does not change with volume or mass changes, thus maintaining a constant molar concentration.
What is molar concentration or molarity?
-Molar concentration, also known as molarity, is the amount of substance (in moles) of a solute divided by the volume of the solution in liters. It is a measure of how much of a substance is present in a given volume of solution.
How does the density of a substance relate to its molar concentration?
-The density of a substance is an intensive property that does not change with the size of the sample. For pure solids and liquids, the density remains constant, which means that the mass per unit volume also remains constant, leading to a constant molar concentration.
Why is the concentration of gases included in the KC expression?
-The concentration of gases is included in the KC expression because it can vary with changes in volume and pressure. Gases do not have a constant density like solids and liquids, and their molar concentration can change with the conditions of the system.
What is the significance of the equilibrium constant (KC) in chemical reactions?
-The equilibrium constant (KC) is a measure of the extent to which a reaction proceeds at equilibrium. It provides information about the relative concentrations of reactants and products at equilibrium, indicating the direction in which the reaction favors.
Can you provide an example of a reaction where the concentration of pure liquids is ignored in the KC expression?
-An example given in the script is the reaction involving ethyl acetate, acetic acid, and ethanol. Water, which is a pure liquid in this context, is not included in the KC expression due to its constant concentration.
What is the difference between an intensive property and an extensive property?
-An intensive property is a characteristic of a substance that does not depend on the amount of material present, such as density or boiling point. An extensive property, on the other hand, depends on the amount of material, such as mass or volume.
Why is the concentration of copper oxide and copper nitrate ignored in the example given?
-Copper oxide and copper nitrate are solid substances, and their concentration is ignored in the KC expression because, as solids, they have a constant density and thus a constant molar concentration that does not affect the equilibrium position.
How does the equilibrium constant (KC) differ from the equilibrium constant in terms of pressure (KP)?
-The equilibrium constant (KC) is expressed in terms of molar concentrations of the reactants and products, while the equilibrium constant in terms of pressure (KP) is expressed in terms of partial pressures. KP is used for reactions involving gases.
What happens to the molar concentration of a substance when its volume changes?
-When the volume of a substance changes, its molar concentration also changes. For gases, an increase in volume leads to a decrease in molar concentration, and vice versa, because the number of moles per unit volume changes.
Can the equilibrium constant (KC) be used to predict the direction of a reaction?
-Yes, the value of KC can be used to predict the direction of a reaction. If KC is greater than 1, the reaction favors the formation of products. If KC is less than 1, the reaction favors the formation of reactants.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowBrowse More Related Video

Kesetimbangan Kimia| Kimia SMA | Tetty Afianti
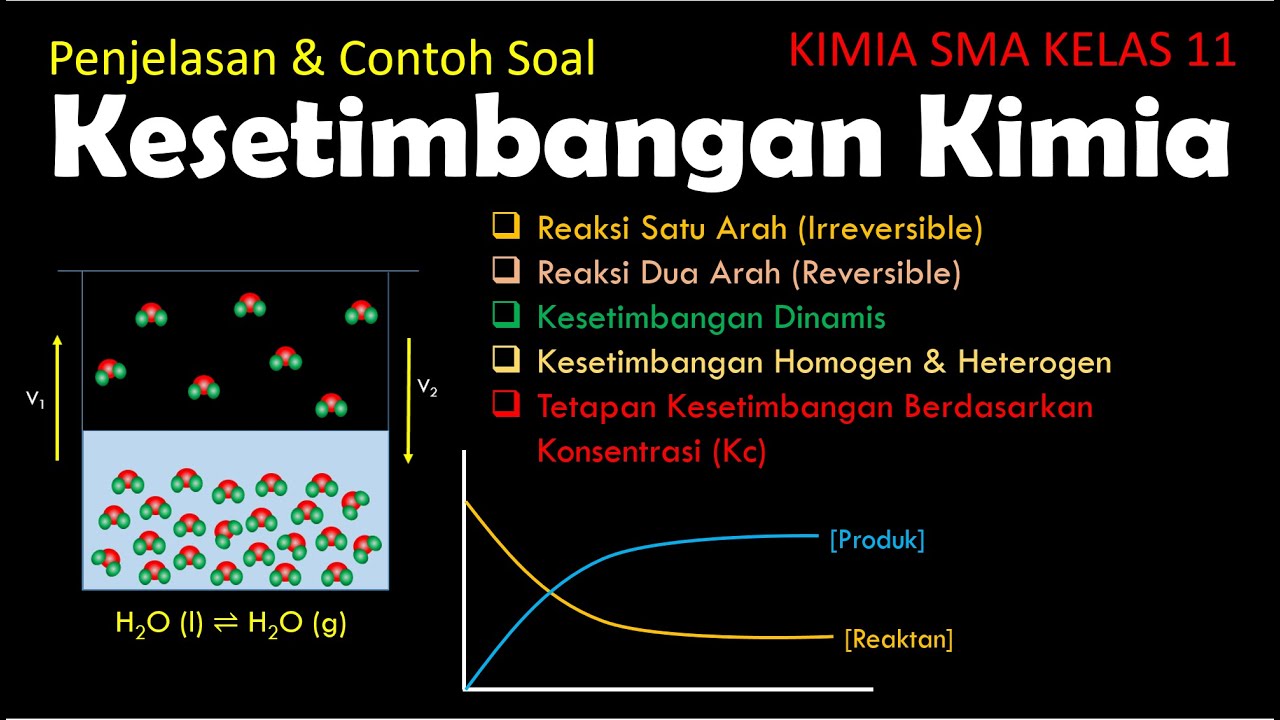
KESETIMBANGAN KIMIA ( KIMIA SMA KELAS 11 )

Part 1 Kesetimbangan Kimia: Reaksi Irreversible, Reaksi Reversible dan Konsep Kesetimbangan Dinamis.

Kesetimbangan Kimia • Part 3: Tetapan Kesetimbangan Pada Reaksi Berkaitan / Hukum Hess

Chemical Equilibria and Reaction Quotients

الكيمياء حسين عبيدة الفصل الثاني 1️⃣
5.0 / 5 (0 votes)