Chemical Equilibria and Reaction Quotients
Summary
TLDRProfessor Dave explores chemical equilibria, explaining that reversible reactions reach a dynamic equilibrium where forward and reverse rates are equal, creating a balance. He introduces ICE (Initial, Change, Equilibrium) tables to calculate concentrations at equilibrium and discusses the equilibrium constant, Kc, which indicates the favorability of products or reactants. The script also covers how to use Kc to predict the direction of a reaction using the reaction quotient, Q, and demonstrates solving for equilibrium concentrations with a sample problem.
Takeaways
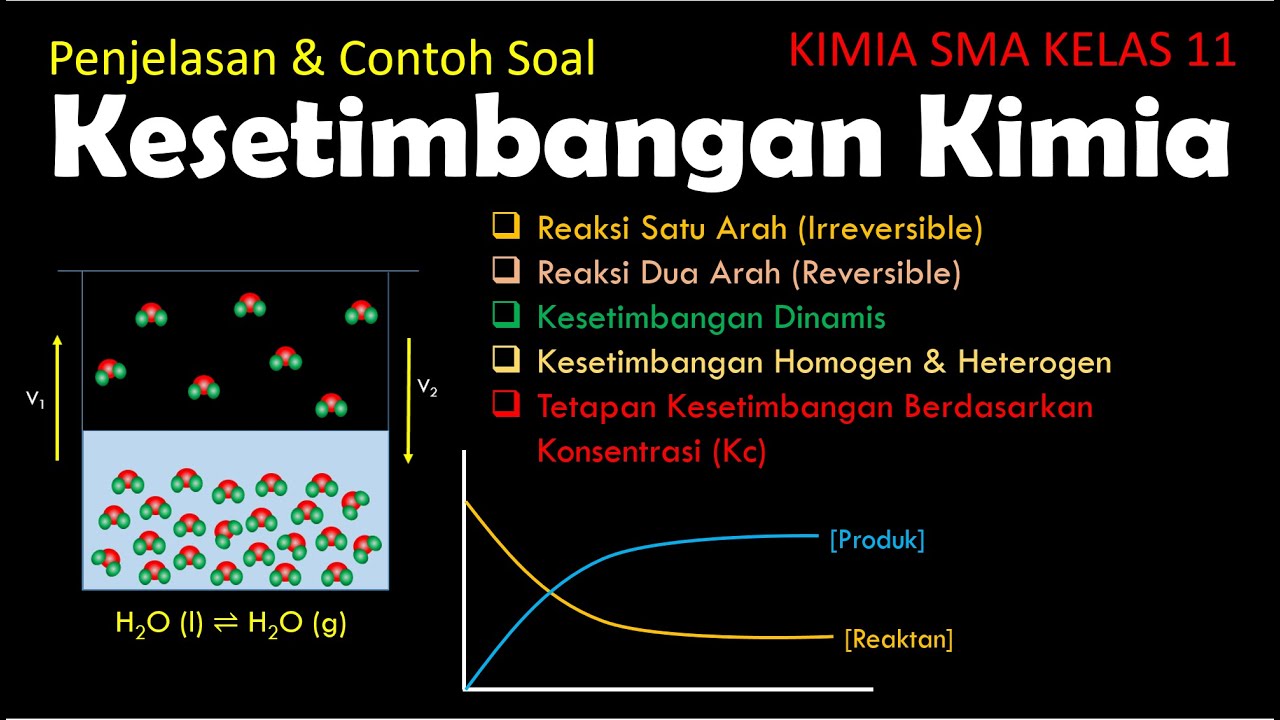
- 🔬 Chemical equilibria involve reversible reactions where reactants and products are interconverted, leading to a dynamic balance where the rates of forward and reverse reactions are equal.
- 🧪 Stoichiometry is used to determine the amounts of products in unidirectional reactions, but equilibrium calculations require additional mathematical approaches due to the reversible nature of the reactions.
- 📊 The ICE (Initial, Change, Equilibrium) table is a method to track the changes in the concentrations of reactants and products as the system reaches equilibrium.
- 📚 The equilibrium constant, Kc, is a ratio of the concentrations of products to reactants raised to their respective stoichiometric coefficients, and it indicates the favorability of products or reactants at equilibrium.
- 📉 A high Kc value (much greater than one) suggests that products are favored in the equilibrium, while a low Kc value (much less than one) indicates that reactants are favored.
- 🌐 Solids and pure liquids are not included in the equilibrium expression as their concentrations are considered constant and not variable like gases or aqueous solutions.
- 🔮 The reaction quotient, Q, is used to predict the direction a reaction will proceed towards equilibrium by comparing non-equilibrium concentrations to the equilibrium constant, Kc.
- 📐 When setting up an ICE table, the change column accounts for the stoichiometric differences between reactants and products, which affects how x (the change in moles) is distributed.
- 🔢 Solving for x in the equilibrium expression can involve taking square roots or, in more complex cases, using the quadratic formula to find the equilibrium concentrations.
- 📘 The equilibrium concentrations of substances are calculated by summing the initial amounts and the changes that occur during the reaction to reach equilibrium.
- 📧 The script encourages viewers to subscribe for more tutorials and reach out with questions, indicating a resource for further learning and clarification.
Q & A
What is a chemical equilibrium?
-A chemical equilibrium is a state in which the rates of the forward and reverse reactions are the same, resulting in no net change in the concentrations of reactants and products over time, even though the reactions continue to occur.
Why is stoichiometry more complicated in equilibrium reactions compared to unidirectional reactions?
-In unidirectional reactions, it's assumed that all reactants are converted to products. In equilibrium reactions, the system involves both the formation and decomposition of products, requiring additional calculations to determine the concentrations of substances at equilibrium.
What does the acronym ICE stand for in the context of equilibrium calculations?
-ICE stands for Initial, Change, and Equilibrium. It's a method used to set up tables to calculate the amounts of reactants and products at equilibrium.
How are the initial amounts represented in an ICE table?
-In an ICE table, the initial amounts are represented by the quantities of substances present before any reaction has occurred. For reactants, this is typically the starting amount, and for products, it's usually zero since they haven't been formed yet.
What is the significance of the equilibrium constant (Kc) in describing an equilibrium system?
-The equilibrium constant (Kc) is a measure of the extent to which a reaction proceeds to completion. It's the ratio of the concentrations of products to reactants, each raised to the power of their stoichiometric coefficients, and indicates whether the reaction favors the formation of products or reactants.
Why are solids and pure liquids not included in the equilibrium constant expression?
-Solids and pure liquids are not included in the equilibrium constant expression because their concentrations are considered constant and do not change during the reaction, thus they do not affect the position of equilibrium.
What is the reaction quotient (Q) and how is it used to predict the direction of a reaction?
-The reaction quotient (Q) is a measure similar to Kc, calculated using the initial concentrations of reactants and products. It's used to predict the direction in which a reaction will proceed to reach equilibrium. If Q < Kc, the reaction will shift towards the products; if Q > Kc, it will shift towards the reactants.
How can you determine the equilibrium concentrations of each substance in a more complex equilibrium system?
-In more complex systems, you can determine the equilibrium concentrations by setting up an ICE table with the correct stoichiometry, calculating the changes in concentrations, and then solving the equilibrium expression for the unknown variable, often x, which represents the change in concentration.
What is the relationship between the equilibrium constant (Kc) and the direction a reaction will shift to reach equilibrium?
-If the reaction quotient (Q) is less than the equilibrium constant (Kc), the reaction will shift towards the products to reach equilibrium. If Q is greater than Kc, it will shift towards the reactants. When Q equals Kc, the system is already at equilibrium.
Why might solving an equilibrium problem require the use of the quadratic equation?
-Solving an equilibrium problem might require the use of the quadratic equation if the equilibrium expression does not simplify in a way that allows for direct calculation of the unknown variable, such as when the powers of the concentrations in the numerator and denominator are not equal.
What is the purpose of the tutorial provided by Professor Dave in the transcript?
-The purpose of the tutorial is to explain the concept of chemical equilibria, how to set up and use ICE tables, understand equilibrium constants, and calculate equilibrium concentrations in various scenarios, including more complex examples.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowBrowse More Related Video
5.0 / 5 (0 votes)