Models of the Atom Timeline
Summary
TLDRThis video script traces the evolution of atomic theory from ancient Greek philosophers to modern quantum mechanics. Democritus and Leucippus first proposed indivisible 'atoms', but Aristotle's four elements theory was more accepted. John Dalton's experiments in 1808 confirmed atoms as basic units. JJ Thompson's discovery of electrons led to the 'plum pudding' model, which was later refined by Rutherford's gold foil experiment revealing a concentrated nucleus. Niels Bohr's planetary model was succeeded by Erwin Schrodinger's quantum mechanical model, depicting electrons as probabilistic orbitals. The script concludes by acknowledging the ongoing refinement of atomic models as scientific understanding deepens.
Takeaways
- 📚 The concept of atoms originated from ancient Greek philosophers Democritus and Leucippus, who proposed that all matter was made of uncuttable particles called 'atomos'.
- 🔬 Aristotle's competing theory of matter being composed of earth, water, air, fire, and ether was more popular at the time, overshadowing the atom theory.
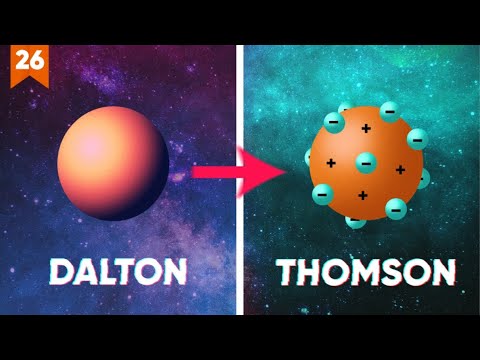
- 🧪 John Dalton introduced the first scientific experiments in 1808, suggesting atoms as indivisible and like tiny balls that combine to form different substances.
- 🌐 J.J. Thompson's discovery of electrons challenged Dalton's model, proposing the 'plum pudding model' where electrons are scattered within a positively charged substance.
- 💥 Ernest Rutherford's gold foil experiment revealed a concentrated positive charge at the atom's center, leading to the 'nuclear model' of the atom.
- 🌀 Niels Bohr's model depicted electrons as planets orbiting a sun-like nucleus, a concept that was later refined by quantum mechanics.
- 🚫 Quantum mechanics, particularly through the work of Erwin Schrodinger, showed that electrons do not orbit in fixed paths but form probabilistic 'orbitals'.
- 🔬 Further discoveries by Rutherford and Chadwick identified protons and neutrons as the subatomic particles within the nucleus.
- 🔍 The quantum mechanical model is the most accurate representation of atoms, though it can be complex for simple explanations.
- 🎨 For simplicity, many educational materials use a hybrid model that combines elements of the Bohr and quantum mechanical models to illustrate atomic structure.
- 🔍 As scientific understanding evolves, the models of atoms are subject to change and refinement to more accurately reflect atomic behavior.
Q & A
Who were the first people to discuss the concept of atoms?
-The first people to discuss the concept of atoms were the ancient Greek philosophers Democritus and his teacher, Leucippus.
What did Democritus believe atoms were?
-Democritus believed that atoms were tiny, uncuttable particles that all matter was made of, and that they came in different sizes and shapes.
What was Aristotle's opposing view to Democritus' atomic theory?
-Aristotle proposed that different things were made of different amounts of the elements earth, water, air, fire, and ether, which was an opposing view to Democritus' atomic theory.
Why did people initially not believe in Democritus' atomic theory?
-People initially did not believe in Democritus' atomic theory because it was not supported by experimental evidence, and Aristotle's ideas were more popular at the time.
Who conducted the first scientific experiments that supported the atomic theory?
-The British chemist John Dalton conducted the first scientific experiments that supported the atomic theory in 1808.
How did John Dalton envision atoms?
-John Dalton envisioned atoms as tiny, indivisible balls that arranged in different combinations to make different substances.
What discovery challenged Dalton's view of atoms being indivisible?
-JJ Thompson's discovery of electrons within atoms challenged Dalton's view, showing that atoms were not indivisible but made up of even smaller particles.
What model of the atom did JJ Thompson propose?
-JJ Thompson proposed the plum pudding model of the atom, where electrons were scattered throughout a positively charged substance, like blueberries in a muffin.
What was Ernest Rutherford's discovery regarding the structure of the atom?
-Ernest Rutherford discovered that atoms have a nucleus where all the positive charge is concentrated, and that atoms are mostly empty space with electrons orbiting around the nucleus.
What was Niels Bohr's model of the atom?
-Niels Bohr proposed a model where electrons orbit the nucleus in circular paths, similar to planets around the Sun.
How did Erwin Schrodinger's work differ from Bohr's model of electron orbits?
-Erwin Schrodinger introduced the concept of electron orbitals, which are regions where electrons are likely to be found, rather than fixed circular orbits.
What are the two subatomic particles that make up the nucleus of an atom?
-The two subatomic particles that make up the nucleus of an atom are protons and neutrons.
What is the current understanding of how electrons behave around the nucleus?
-The current understanding is that electrons do not orbit the nucleus in fixed paths but rather move in a way that can be described by quantum mechanics, forming orbitals of various shapes.
Why might the video use a simplified model of the atom for certain explanations?
-The video might use a simplified model of the atom to make it easier to convey fundamental topics and concepts, even though it is not an exact representation of the quantum mechanical model.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowBrowse More Related Video
5.0 / 5 (0 votes)