Energy levels, sublevels, & orbitals
Summary
TLDRThis video explores the structure of atoms with a focus on electrons, energy levels, and orbitals. It begins by explaining the significance of atomic numbers and how electrons are arranged in hydrogen, carbon, and sodium atoms. Using a hotel analogy, the video illustrates energy levels as floors, sublevels as rooms, and orbitals as beds where electrons reside. It further connects these concepts to the periodic table, detailing how electron configurations influence elemental properties. This engaging overview provides essential insights into atomic theory and the organization of elements in chemistry.
Takeaways
- 😀 Hydrogen has an atomic number of 1, indicating it has one proton and one electron, and is a neutral atom.
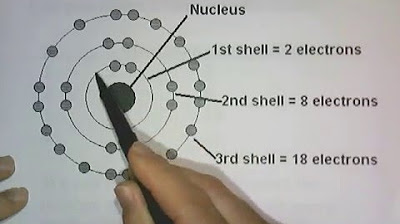
- 😀 The first energy level (ground state) can hold a maximum of 2 electrons, while subsequent levels can hold up to 8 electrons.
- 😀 Carbon, with an atomic number of 6, has 2 electrons in the first energy level and 4 in the second.
- 😀 Sodium has an atomic number of 11, with electrons distributed across three energy levels: 2 in the first, 8 in the second, and 1 in the third.
- 😀 Valence electrons are found in the outermost energy level of an atom and are crucial for determining the atom's chemical behavior.
- 😀 Energy levels in an atom can be compared to floors in a hotel, with sublevels representing rooms and orbitals representing beds.
- 😀 The sublevels 's', 'p', 'd', and 'f' define where electrons can reside, with each having a different number of orbitals: 's' has 1, 'p' has 3, 'd' has 5, and 'f' has 7.
- 😀 The arrangement of the periodic table reflects the sublevels, with the first two columns corresponding to 's', transition metals to 'd', and elements on the right to 'p'.
- 😀 The total number of electrons that can fit in each energy level increases with the level number, allowing for more complex electron configurations.
- 😀 Understanding electron configurations helps explain the properties and behaviors of elements based on their placement in the periodic table.
Q & A
What is the primary focus of the video?
-The video focuses on the location of electrons in an atom and how they are organized within energy levels, sublevels, and orbitals.
What does the atomic number represent in an atom?
-The atomic number represents the number of protons in an atom, and for a neutral atom, it also equals the number of electrons.
How many electrons can the first energy level hold?
-The first energy level can hold a maximum of two electrons.
What is the electron configuration for a neutral carbon atom?
-A neutral carbon atom, with an atomic number of six, has two electrons in the first energy level and four electrons in the second energy level, giving it a configuration of 2, 4.
What are valence electrons, and where are they found?
-Valence electrons are the electrons in the outermost energy level of an atom. They are important for chemical bonding and reactivity.
What analogy does the video use to explain energy levels and orbitals?
-The video uses the analogy of a hotel, where energy levels are the floors, sublevels are the rooms, and orbitals are the beds in those rooms.
How are the sublevels organized within energy levels?
-Sublevels within energy levels are organized as follows: the first level has one sublevel (s), the second has two (s and p), the third has three (s, p, and d), and the fourth has four (s, p, d, and f).
What is the maximum number of electrons that can occupy the d sublevel?
-The d sublevel has five orbitals, and since each orbital can hold two electrons, the maximum number of electrons in the d sublevel is 10.
What pattern does the periodic table follow concerning sublevels?
-The periodic table is arranged in blocks that correspond to the sublevels: the first two columns are the s block, the middle transition metals are the d block, and the elements on the right are primarily in the p block.
What is the significance of the number of orbitals in each sublevel?
-The number of orbitals in each sublevel determines how many electrons can occupy that sublevel, which in turn influences the chemical properties of the element.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowBrowse More Related Video
Energy Levels, Energy Sublevels, Orbitals, & Pauli Exclusion Principle

3.6.3 - Como fazer a distribuição eletrônica dos elétrons de um átomo: hidrogênio, hélio e lítio
stuktur atom

Aufbau's Principle, Hund's Rule & Pauli's Exclusion Principle - Electron Configuration - Chemistry

3.5.1 - Introdução ao conceito de orbital e números quânticos

Structure of an Atom
5.0 / 5 (0 votes)