S1.3.3 / S1.3.4 Atomic orbitals and sub-levels
Summary
TLDRThis video offers an insightful look into atomic orbitals and their sublevels. It explains that s orbitals are spherical, while p orbitals are dumbbell-shaped and oriented at 90 degrees to each other. The script delves into the principal energy levels, detailing the number of sublevels and electrons each can hold, from the 1s sublevel with 2 electrons to the 4f sublevel with 14, culminating in a total of 32 electrons for the n=4 level. The video promises to explore the electron filling order in the next installment, piquing interest for further learning.
Takeaways
- 🌐 An atomic orbital is defined as a region of space where there is a high probability of finding an electron.
- 📘 S orbitals are spherical in shape, with examples being the 1s and 2s orbitals.
- 🔄 P orbitals are dumbbell-shaped and exist in three orientations: px, py, and pz, which are at 90 degrees to each other.
- 📚 Principal energy levels are split into sublevels, with the number of sublevels increasing as the principal quantum number (n) increases.
- 🔢 The first principal energy level (n=1) has one sublevel, the 1s sublevel.
- 📈 The second principal energy level (n=2) has two sublevels, the 2s and 2p sublevels.
- 📊 The third principal energy level (n=3) has three sublevels, the 3s, 3p, and 3d sublevels.
- 🎓 The fourth principal energy level (n=4) has four sublevels, the 4s, 4p, 4d, and 4f sublevels.
- 📉 The energy of sublevels within a principal energy level increases from s to f, with the s sublevel being the lowest and the f sublevel being the highest.
- 🧠 The order of filling of atomic orbitals with electrons will be discussed in the next video.
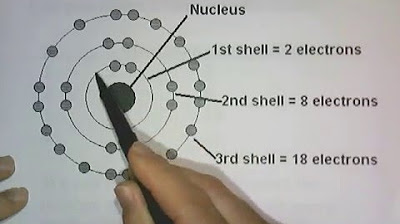
- 📊 A summary table in the video provides the number of sublevels, orbitals, and electrons for each principal energy level, with the maximum number of electrons for n=1 being 2, n=2 being 8, n=3 being 18, and n=4 being 32.
Q & A
What is an atomic orbital?
-An atomic orbital is a region of space where there is a high probability of finding an electron.
What are the shapes of s orbitals?
-S orbitals are spherical in shape.
How many s orbitals are there in the first principal energy level?
-There is one s orbital in the first principal energy level, known as the 1s orbital.
What is the shape of p orbitals?
-P orbitals are dumbbell-shaped.
How many p orbitals are there in the p sublevel?
-There are three p orbitals in the p sublevel, labeled as px, py, and pz.
What is the orientation of the p orbitals relative to each other?
-The p orbitals are oriented at 90 degrees to each other.
What are the sublevels of the second principal energy level?
-The second principal energy level has two sublevels: the 2s and the 2p sublevels.
How many sublevels does the third principal energy level have?
-The third principal energy level has three sublevels: the 3s, 3p, and 3d sublevels.
What is the order of energy for the sublevels within the main energy level?
-Within the main energy level, the order of energy is from lowest to highest: s, p, d, f.
What is the maximum number of electrons the 3d sublevel can hold?
-The 3d sublevel can hold a maximum of 10 electrons.
How many electrons can the 4f sublevel hold and what is its energy level?
-The 4f sublevel can hold a maximum of 14 electrons and it is part of the fourth principal energy level.
What is the total number of electrons that the fourth principal energy level can hold?
-The fourth principal energy level can hold a maximum of 32 electrons.
What will be the focus of the next video after the one described in the script?
-The next video will focus on the order of filling these atomic orbitals with electrons.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowBrowse More Related Video

3.5.4 - Composição do orbital atômico: Número quântico secundário (subcamada ou subnível de energia)
Energy Levels, Energy Sublevels, Orbitals, & Pauli Exclusion Principle

Energy levels, sublevels, & orbitals

3.5.5 - Composição do orbital atômico: Número quântico magnético (Orientação espacial dos orbitais)

3.6.3 - Como fazer a distribuição eletrônica dos elétrons de um átomo: hidrogênio, hélio e lítio

Shells-Subshells-Orbitals
5.0 / 5 (0 votes)