GCSE Physics - Specific Latent Heat #29
Summary
TLDRThis video explores the concept of specific latent heat (SLH), which is the energy required to change a substance's state without altering its temperature. It explains how SLH differs from the typical increase in temperature when heating a substance, highlighting the processes of melting, freezing, evaporation, and condensation. The video uses the example of heating one kilo of ice to water vapor, detailing the specific latent heat of fusion and vaporization for water, and provides the formula for calculating energy changes during state transitions. It concludes with a practical application question involving boiling water.
Takeaways
- 🔥 The concept of specific latent heat is introduced, which is the energy required to change a substance's state without changing its temperature.
- 🌡️ Temperature is a measure of the average internal energy of particles in a substance, but it doesn't increase during state changes due to energy being used to break inter-particle forces.
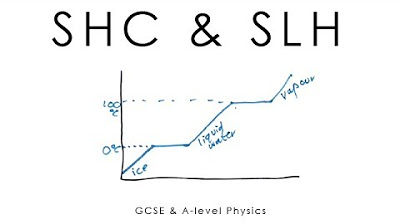
- 📊 The graph of temperature change over time for a substance is not a straight line, especially during phase transitions where energy input is used to change states, not increase temperature.
- ❄️ The specific latent heat of fusion is the energy required to change a substance from a solid to a liquid, such as ice melting into water.
- 🌫️ The specific latent heat of vaporization is the energy required for a substance to change from a liquid to a gas, like water boiling into vapor.
- 💧 For water, the specific latent heat of fusion is 334,000 joules per kilogram, which is the energy needed to melt ice at 0 degrees Celsius.
- 🌬️ The specific latent heat of vaporization for water is 2,260,000 joules per kilogram, necessary to convert liquid water into water vapor at 100 degrees Celsius.
- 🔄 The same principles apply in reverse during cooling, with energy being released during state changes, which keeps the temperature constant.
- ⚖️ The specific latent heat (SLH) is defined as the energy required to change one kilogram of a substance from one state to another without a temperature change.
- 📘 The formula for calculating specific latent heat is the mass of the substance multiplied by the specific latent heat value.
- 📝 An example calculation is provided to demonstrate how to use the specific latent heat values to determine the energy required to boil a certain mass of water.
Q & A
What is specific latent heat?
-Specific latent heat is the amount of energy required to change the state of a substance while the temperature remains constant. It is used to break or form bonds between particles during phase changes such as melting, freezing, vaporization, or condensation.
Why does the temperature of a substance remain constant during a phase change?
-The temperature remains constant during a phase change because the energy supplied is used to overcome the forces between particles rather than to increase their kinetic energy, which is what typically raises temperature.
What are the two types of specific latent heat mentioned in the script?
-The two types of specific latent heat are the specific latent heat of fusion, which is the energy change when a substance changes between a solid and a liquid, and the specific latent heat of vaporization, which is the energy change when a substance changes between a liquid and a gas.
What is the specific latent heat of fusion for water?
-The specific latent heat of fusion for water is 334,000 joules per kilogram, which is the energy required to change ice at 0 degrees Celsius to liquid water without changing its temperature.
What is the specific latent heat of vaporization for water?
-The specific latent heat of vaporization for water is 2,260,000 joules per kilogram, which is the energy required to change liquid water at 100 degrees Celsius to water vapor without changing its temperature.
How is the specific latent heat used in calculations?
-The specific latent heat is used in calculations to determine the amount of energy required or released during a phase change. It is calculated using the formula: Energy = Mass of the substance × Specific latent heat.
What happens to the temperature of a substance when it is heated from a solid state to a gaseous state, passing through a liquid state?
-As the substance is heated from a solid to a liquid state, the temperature remains constant at the melting point until all the substance has melted. Then, as it is heated from a liquid to a gas, the temperature remains constant at the boiling point until all the substance has vaporized.
What is the difference between the energy required for a substance to melt and the energy released when it freezes?
-The energy required for a substance to melt is the specific latent heat of fusion, which is absorbed to break the bonds between particles. Conversely, the energy released when a substance freezes is the same amount of energy, but it is released as the substance changes from a liquid to a solid state.
How can you calculate the energy required to boil a certain amount of water?
-To calculate the energy required to boil water, you multiply the mass of the water by the specific latent heat of vaporization. For example, to boil 2.5 kilograms of water, you would use the formula: 2.5 kg × 2,260,000 J/kg = 5,650,000 J or 5,650 kilojoules.
Why is it important to understand specific latent heat in the context of phase changes?
-Understanding specific latent heat is important because it helps explain why the temperature of a substance does not change during phase changes, despite continuous energy input or output. It is also crucial for various applications in science and engineering, such as refrigeration, heating, and thermal energy storage.
What is the significance of the graph in the script that shows how the temperature of a substance changes with time as it is heated?
-The graph is significant because it illustrates the non-linear relationship between temperature and heat input during phase changes. It shows that the temperature plateaus at the melting and boiling points, indicating that the energy is being used for phase change rather than increasing temperature.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowBrowse More Related Video

Calor Sensible y Calor Latente |Termodinámica| - Salvador FI
Specific Heat Capacity + Latent Heat - GCSE & A-level Physics (full version)

Materi Kalor Kelas 7 SMP

GCSE Physics - Internal Energy and Specific Heat Capacity #28
Termologia | Calorimetria - Parte I (RESUMÃO)

Physics 22 Introduction to Heat & Temperature (6 of 6) Change of Phase & Latent Heat
5.0 / 5 (0 votes)