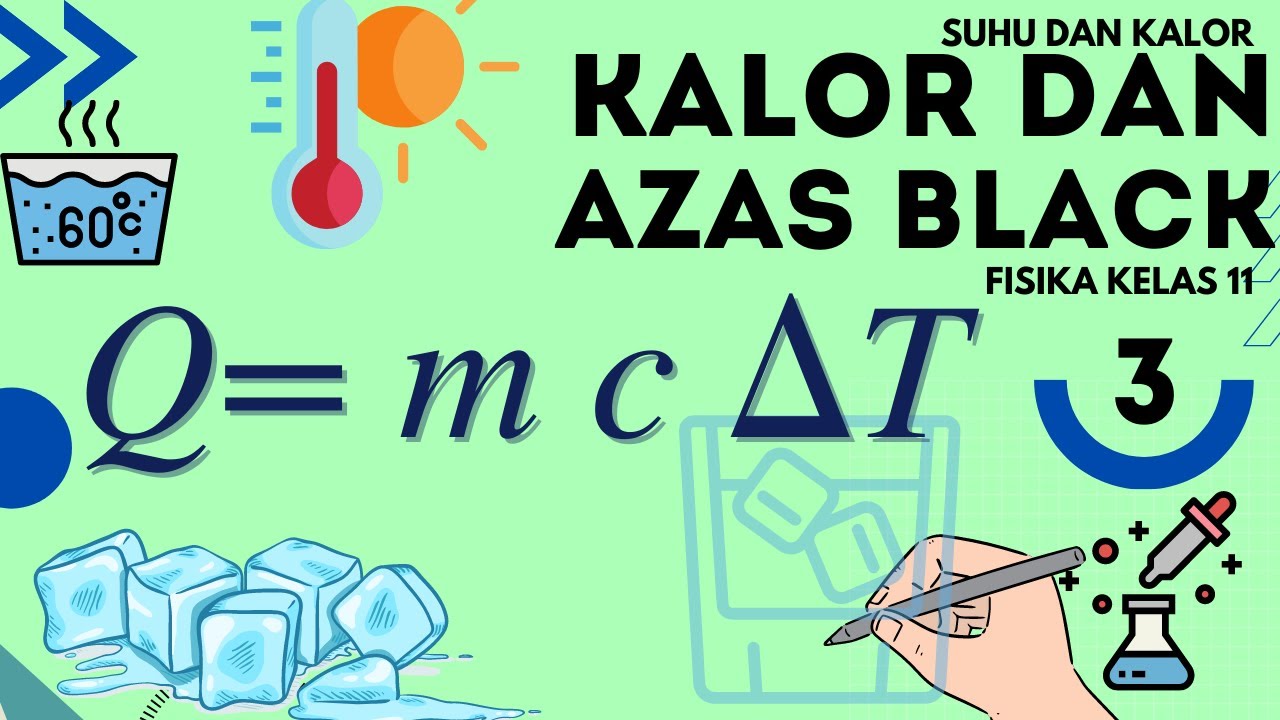TROCAS DE CALOR COM MUDANÇA DE FASE - TERMOLOGIA - Aula 8 - Prof. Boaro
Summary
TLDRIn this educational video, the teacher explains key concepts in thermal physics, specifically focusing on heat exchange with and without phase changes. They describe the process of heat transfer between substances, such as ice melting in water, and illustrate how temperature varies during these exchanges. The video also walks through a practical example of calculating the latent heat of fusion using a calorimeter, offering insights into important formulas and concepts in thermodynamics. The teacher provides both theory and practical applications to help students grasp the material effectively.
Takeaways
- 😀 Heat exchange occurs without a phase change in some cases, where substances only exchange heat between themselves without altering their physical state.
- 😀 When a substance undergoes a phase change (like melting or vaporization), the heat equation changes, with mass and latent heat playing significant roles.
- 😀 The latent heat of fusion (80 cal/g) and latent heat of vaporization (540 cal/g) for water are key reference values for calculations involving water.
- 😀 A calorimeter is a device used to measure heat exchange, where substances are placed in a container to achieve thermal equilibrium.
- 😀 In heat transfer problems, the temperature-time or heat versus temperature graphs can be used to track the progress of heat exchange.
- 😀 When mixing substances like ice at -18°C and water at 80°C, the ice must first warm up to 0°C before it starts to melt.
- 😀 During the melting of ice, there is no change in temperature until all the ice has melted, despite heat being exchanged.
- 😀 The final thermal equilibrium temperature of substances, like ice and water, depends on their mass and specific heat capacities.
- 😀 The heat capacity of water (1 cal/g°C) and the latent heat of fusion are critical for solving problems involving the melting of ice and heating of water.
- 😀 In exercises involving heat exchange, the formula involves the mass of water, the heat capacity, and temperature changes, along with the latent heat of fusion for phase changes.
Q & A
What is the main topic of the lesson in this script?
-The main topic of the lesson is thermodynamics, specifically focusing on heat transfer with and without a phase change in substances.
What is the key difference between heat transfer with and without a phase change?
-Heat transfer without a phase change involves substances exchanging heat without altering their state of aggregation, while heat transfer with a phase change involves a substance undergoing a change in state, such as from solid to liquid or liquid to gas.
What formula is used to calculate the heat transferred when there is no phase change?
-The formula used is: Q = m × c × ΔT, where Q is the heat transferred, m is the mass of the body, c is the specific heat capacity, and ΔT is the change in temperature.
What is the formula for heat transfer when there is a phase change?
-The formula used is: Q = m × L, where Q is the heat transferred, m is the mass of the substance, and L is the latent heat (which can be the latent heat of fusion or latent heat of vaporization depending on the phase change).
What are the latent heat values for water mentioned in the lesson?
-The latent heat of fusion for water is 80 calories per gram, and the latent heat of vaporization for water is 540 calories per gram.
What is the role of a calorimeter in heat transfer experiments?
-A calorimeter is used to measure the heat transferred between substances in an isolated system, ensuring that the heat exchange occurs only between the substances inside the calorimeter without external interference.
What does the horizontal part of the temperature graph represent?
-The horizontal part of the temperature graph represents a phase change, where the temperature remains constant as a substance changes its state, such as when ice melts or water boils.
What happens when ice at -18°C is placed in water at 80°C, according to the example?
-The ice will first absorb heat to reach 0°C, then it will melt and continue to absorb heat without a change in temperature until it is completely melted, at which point the water's temperature decreases.
What is the specific question in the exercise that the professor discusses?
-The exercise asks to determine the latent heat of fusion of ice when a known mass of ice at 0°C is added to a mass of water at 19.8°C, with the final temperature reaching 0°C and the ice completely melting.
How is the latent heat of fusion calculated in the example provided?
-The calculation involves using the heat transfer formula for water and ice, considering that the heat lost by the water is equal to the heat gained by the ice, which includes the latent heat of fusion to melt the ice.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowBrowse More Related Video
5.0 / 5 (0 votes)