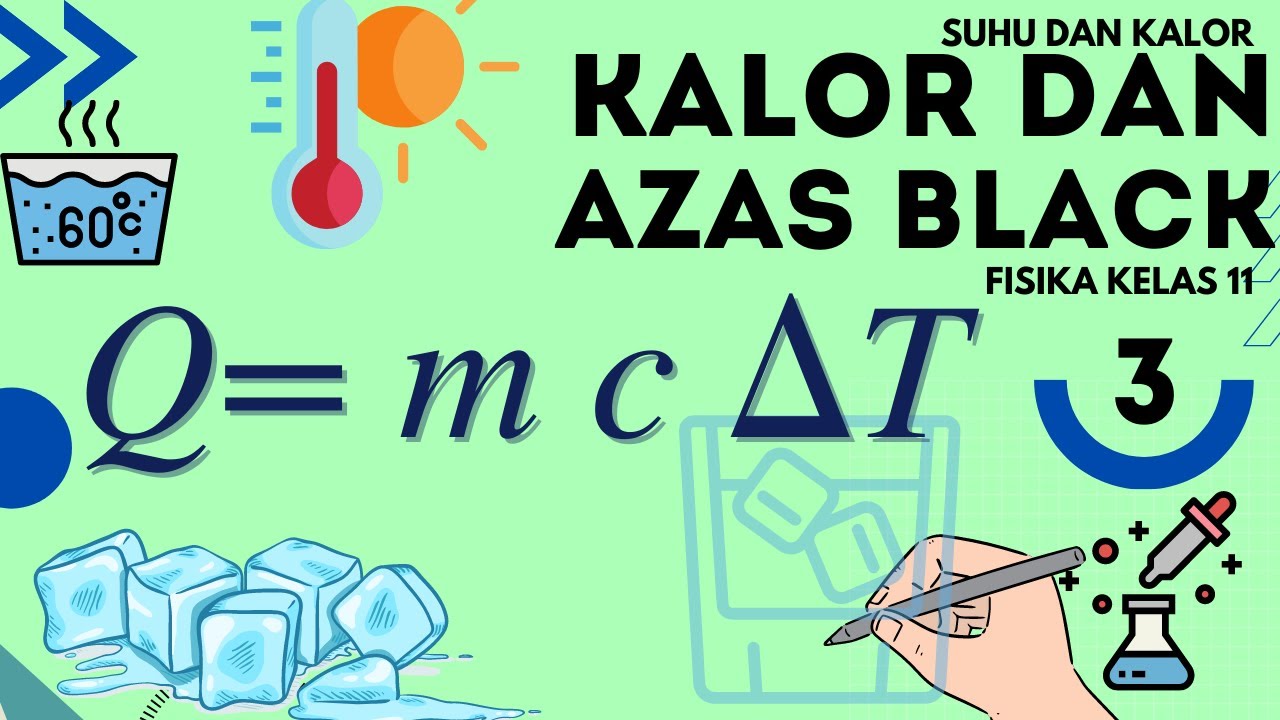KALOR | Suhu dan Kalor - Fisika Kelas 11
Summary
TLDRIn this educational video, the presenter explains concepts related to temperature and heat, particularly focusing on thermal expansion and calorimetry. The video covers the principles of heat transfer, the phase changes of water (from solid to liquid to gas), and how to calculate the heat energy involved in these transformations using formulas like Q = mcΔT and Q = mL. Additionally, the presenter discusses real-life applications of these principles, such as heating water and mixing different temperatures of substances. The video also includes practical examples, making complex physics concepts more relatable and understandable.
Takeaways
- 😀 Calor (heat) can originate from fire or electricity, and it plays a crucial role in changing the state of matter, like ice turning to water and water turning to steam.
- 😀 When a substance is given heat, it can either raise its temperature or cause a phase change, such as from solid to liquid or liquid to gas.
- 😀 The formula for calculating heat (calor) when temperature changes is Q = m * c * ΔT, where m is mass, c is specific heat capacity, and ΔT is the change in temperature.
- 😀 When heat is applied to ice, its temperature increases until it reaches 0°C, after which it melts into water without any temperature increase during the phase change.
- 😀 The process of water boiling involves the temperature rising to 100°C before turning into steam, and no temperature increase occurs during the phase change from liquid to gas.
- 😀 After water reaches 100°C and begins to boil, the temperature remains constant until all of the water has vaporized into gas, even if heating continues.
- 😀 The phase diagram of water illustrates the transitions between solid (ice), liquid (water), and gas (steam), and these transitions happen at specific temperatures under normal conditions.
- 😀 According to Black's principle, when two substances at different temperatures are mixed, the heat lost by the hotter substance is equal to the heat gained by the cooler one, provided no heat is lost to the environment.
- 😀 The video explains the use of Black's principle to calculate the final temperature when two substances are mixed, such as hot tea with ice or hot and cold water.
- 😀 In a practical example, a calculation is made where 200g of water at 10°C is mixed with 100g of water at 70°C, and the final temperature of the mixture is determined using heat transfer equations.
Q & A
What is the relationship between heat and temperature in a substance?
-Heat is energy that flows from a hotter object to a cooler one, causing a change in temperature or phase. When heat is absorbed by a substance, its temperature can increase, or it can undergo a phase change without a temperature change.
What is the formula used to calculate the heat energy when the temperature of a substance changes?
-The formula is Q = m * c * ΔT, where Q is the heat energy, m is the mass of the substance, c is the specific heat capacity, and ΔT is the change in temperature.
Why does the temperature of a substance remain constant during a phase change, like melting or boiling?
-During a phase change, such as melting or boiling, the added heat energy is used to overcome the forces between particles, changing the substance's state without increasing its temperature until the phase transition is complete.
What is the formula for calculating the heat required for a substance to change phase?
-The formula is Q = m * L, where Q is the heat energy, m is the mass of the substance, and L is the latent heat of fusion (for melting) or latent heat of vaporization (for boiling).
How does the heat energy change when a substance melts, for example, ice turning into water?
-When ice melts, heat energy is absorbed to break the bonds between molecules, causing the phase change from solid to liquid. The temperature remains constant at 0°C during the melting process until all the ice has turned into water.
Why does water remain at 100°C during boiling even if it is continuously heated?
-During boiling, the heat energy added is used to turn the liquid into vapor. The temperature remains constant at 100°C as the water changes from liquid to gas. Only after all the water has evaporated can the temperature increase further.
What does the principle of calorimetry explain when mixing hot and cold substances?
-Calorimetry explains that when hot and cold substances are mixed, the heat lost by the hot substance is equal to the heat gained by the cold substance, assuming no heat is lost to the environment. This principle allows us to calculate the final temperature of the mixture.
How do you calculate the final temperature when mixing water at different temperatures?
-To calculate the final temperature, apply the equation Q = m * c * ΔT for each substance, setting the heat lost by the hot water equal to the heat gained by the cold water. Solve for the final temperature, considering the masses and specific heat capacities of the substances.
What is the importance of specific heat capacity in heating substances?
-Specific heat capacity is the amount of heat required to raise the temperature of a substance by 1°C. It helps determine how much heat a substance will absorb or release when its temperature changes, and it varies from one material to another.
What does the phase diagram of water show, and why is it important?
-The phase diagram of water shows the relationship between temperature, pressure, and the phases of water (solid, liquid, and gas). It helps understand how water behaves under different conditions and is useful in predicting phase transitions such as freezing, melting, and boiling.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade Now5.0 / 5 (0 votes)